doi: 10.56294/mw20227
SYSTEMATIC REVIEW
Interoperability standards in Health Information Systems
Estándares de interoperabilidad en los Sistemas de Información en Salud
Carlos Canova-Barrios1 ![]() *,
Felipe Machuca-Contreras2
*,
Felipe Machuca-Contreras2 ![]() *
*
1Universidad de Ciencias Empresariales y Sociales. Ciudad Autónoma de Buenos Aires, Argentina.
2Universidad Autónoma de Chile, Santiago, Chile.
Cite as: Canova-Barrios C, Machuca-Contreras F. Interoperability standards in Health Information Systems: systematic review. Seminars in Medical Writing and Education 2022; 1:7. https://doi.org/10.56294/mw20227.
Submitted: 05-04-2022 Revised: 12-07-2022 Accepted: 16-08-2022 Published: 17-08-2022
Editor: Dr.
José Alejandro Rodríguez-Pérez ![]()
ABSTRACT
Introduction: Health Information Systems (HIS) have been improved to enhance the quality of healthcare. However, adequate computerization of these systems, as demanded by the digital society, requires the adoption of interoperability standards that allow all system elements to be interconnected.
Objective: to describe the results provided by the literature on the use of interoperability standards in HIS.
Methods: a systematic review was conducted using the PRISMA methodology on the results of the adoption of interoperability standards in HIS. Information was searched in Scopus, Medline, Google Scholar, and SciELO. Then, a selection of the most relevant studies was made, using inclusion criteria.
Results: most of the reviewed studies focused on the evaluation of interoperability standards in hospital and institutional environments, with a global interest in such standards. The diversity of standards used and the results obtained demonstrate the importance and significant impact of the implementation of interoperability standards in improving the quality of healthcare systems.
Conclusions: the systematic literature review reveals that the implementation of interoperability standards is fundamental to ensure the integration between the components that make up the systems, which in turn contributes to operational efficiency and information security.
Keywords: Interoperability Standards; Interoperability; Health Information Systems.
RESUMEN
Introducción: los Sistemas de Información en Salud (SIS) se han perfeccionado para mejorar la calidad de la atención médica. Sin embargo, una adecuada informatización de estos sistemas, tal y como demanda la sociedad digital, requiere de la adopción de estándares de interoperabilidad que permitan interconectar todos los elementos del sistema.
Objetivo: describir los resultados aportados por la literatura de la utilización de estándares de interoperabilidad en los SIS.
Métodos: se realizó una revisión sistemática mediante la metodología PRISMA sobre los resultados de la adopción de estándares de interoperabilidad en SIS. Se partió de una búsqueda de información en Scopus, Medline, Google Scholar y SciELO. Luego se realizó una selección de los estudios más pertinentes, mediante la aplicación de criterios de inclusión.
Resultados: la mayoría de los estudios revisados se enfocaron en la evaluación de estándares de interoperabilidad en entornos hospitalarios e institucionales, con un interés global por dichos estándares. La diversidad de estándares utilizados y los resultados obtenidos demuestran la importancia y el impacto significativo de la implementación de estándares de interoperabilidad en la mejora de la calidad de los sistemas de salud.
Conclusiones: la revisión sistemática de literatura revela que la implementación de estándares de interoperabilidad es fundamental para garantizar la integración entre los componentes que conforman los sistemas, lo que a su vez contribuye a la eficiencia operativa y la seguridad de la información.
Palabras clave: Estándares de Interoperabilidad; Interoperabilidad; Sistemas de Información en Salud.
INTRODUCTION
Every society needs reliable and quality health care for its structural functioning. Information is an indispensable resource in guaranteeing the quality of health services. It is a fundamental asset for the mechanisms that support healthcare administration, understood as Information Systems, which are managed with greater intentionality using technological tools.
For the World Health Organization (WHO), the Health Information System (HIS) is the set of integrated elements for collecting, processing, analyzing, and transmitting information needed to organize and operate health services, research, and planning to control health events.(1) They allow immediate access to and exchange information for decision-making. The SIS has been refined to improve the quality of care from the patient's perspective as the system's most relevant element.(2) In previous stages, the SIS focused more on supporting the administration and management of goods and resources. The paradigms oriented to the proactive and preventive care model direct health information management.
It promotes the need for efficient SIS in management, which ensures tensuringeliness, integrity, and absolute reliability of information to improve the quality of services. In this healthcare demand, the implementation of technologies has shown great feasibility.
According to the literature,(3,4) Modern, computerized healthcare systems have allowed the practice of theoretical knowledge and improvements in skills and abilities to benefit the quality of healthcare systems.
Implementing a computerized information system is beneficial for institutions, patients, and information users in general. It is essential to design the system in such a way that it meets its quality objectives.(5) Information technologies bring competitiveness to processes and drive the development of information management platforms. There is evidence of the impact of this transformation on the prevention, treatment, and diagnosis of health problems in society.(3,4)
The technological facilities in the SIS have allowed the development of electronic clinical records and electronic medical records (EHR), which have improved the doctor-patient relationship. These tools offer advantages in reducing time spent on generating and consuming information compared to traditional paper records.(4,6)
This type of information system has experienced a gradual increase in its implementation in hospitals worldwide, providing efficiency, quality, and satisfaction in patient care. The information managed through these systems favors communication between different institutions and levels of health care for a more comprehensive approach to the care needs of the population. These technologies aim to link data at the local and national level through a single patient medical record available at any time and place where care is required.(7)
In this task, the interoperability of the SIS takes on significant importance. It represents a critical factor in speeding up the exchange of information between healthcare entities in a collaborative environment. It is an elementary component for developing digital societies by creating new value through innovation, thus maximizing the benefits of information technologies for health.
In this sense, interoperability is defined as the ability of two or more systems or components to exchange information and use the information that has been exchanged.(8,9,10) Interoperability occurs when different applications and digital platforms can exchange data, interpret it, and make it readily available to users and stakeholders.(11)
However, achieving interoperability is a complex process and is one of the biggest challenges in computerized SIS. Interoperability requires standards, which are norms and specifications that allow different systems to exchange data effectively and automatically. They are essential to ensure integration between system components and systems, contributing to operational efficiency and information security.(12)
Indeed, applying information technologies to SIS and the interoperability of systems is a technological and legal challenge, especially in decentralized environments. This leads to the need to go deeper into interoperability standards by analyzing experiences in their implementation.
This article aims to describe the results provided by the literature on the use of interoperability standards in Health Information Systems, based on the boom obtained by them in the health sector parallel to the development of information systems and information and communication technologies.
METHODS
A systematic literature review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology.(13,14) The review aims to synthesize evidence on the results of adopting interoperability standards in SIS and the conditions of such implementation. The study design was based on the research conducted by Ovies-Bernal et al.(15)
This review was based on a search for information in different bibliographic database systems, indexing and abstracting systems, and search engines (Table 1).
|
Table 1. Databases consulted and search strategies employed |
|
|
Databases |
Search Strategy |
|
Scopus |
TITLE-ABS-KEY ("Interoperability") AND DOCTYPE ("ar") AND (LIMIT-TO (SUBJAREA, "MEDI") OR LIMIT-TO (SUBJAREA, "HEAL")) |
|
SciELO |
AND (Interoperability) AND (Health Information Systems) (Interoperability) AND (Health Information Systems) |
|
Google Schoolar |
|
|
PubMed/Medline |
("Interoperabilidad"[Título/resumen] AND "sistemas de información sanitaria"[Título/resumen]) |
In order to meet the objective, we included those studies, preferably from the last five years, published in scientific journals that met the following criteria:
· Articles on interoperable SIS implementations
· Studies where messaging, terminology, or document standards have been implemented
· Research with results on the adoption of such standards
· Studies stating which standards were used
· Publications in English or Spanish.
So-called "gray literature" sources and government documents were not included. In cases where exciting results were found from these sources, manual searches were performed to check other sources that specifically reported information about these results.
Studies identified as a result of the searches in the different databases were checked for duplicity, and duplicate records were eliminated. Studies were screened according to title and abstract, and subsequently, the full texts of the articles were reviewed.
Papers that only addressed the IT-technical aspect of system implementation or that, on the contrary, focused on the regulatory aspect without considering the IT aspects were discarded. This way, we avoided selecting documents that only covered one component or were partial or biased.
Unlike the study by Ovies-Bernal et al.(15) we did not exclude studies that presented implementation only at the hospital level or were limited to processing electronic clinical records. These studies can also provide value regarding the experience of computerizing information systems.
RESULTS AND DISCUSSION
The information search made it possible to retrieve a total of 16018 sources of information. Most of these were obtained through Google Scholar (89,3 %). This search engine was followed by Scopus (9,2 %), Pubmed (1,2 %), and SciELO (0,3 %). However, a search with such broad results had much information irrelevant to the study. Through applying filters (period 2018-2023, open access, journal articles, original articles) and eliminating matching records in the different databases, the search results were simplified to a total of 211 studies.
From this first screening, it was possible to exclude 89 studies (42,2 % of the articles included in this stage) from the 211 mentioned above. With this exclusion, 122 articles passed to the suitability selection stage. At this stage, 66 (54,1 %) articles were eliminated by analyzing their summaries. Of the remaining 56 (45,9 %), 36 (29,5 %) were eliminated after a critical reading of the full text of the articles.
Finally, it was decided to include 20 articles that met the stated inclusion criteria and the quality parameters necessary to be included in the literature review. This amount represents 0,1 % of the total number of articles in the information search. The selection process was described in the flow chart of this review (Figure 1).
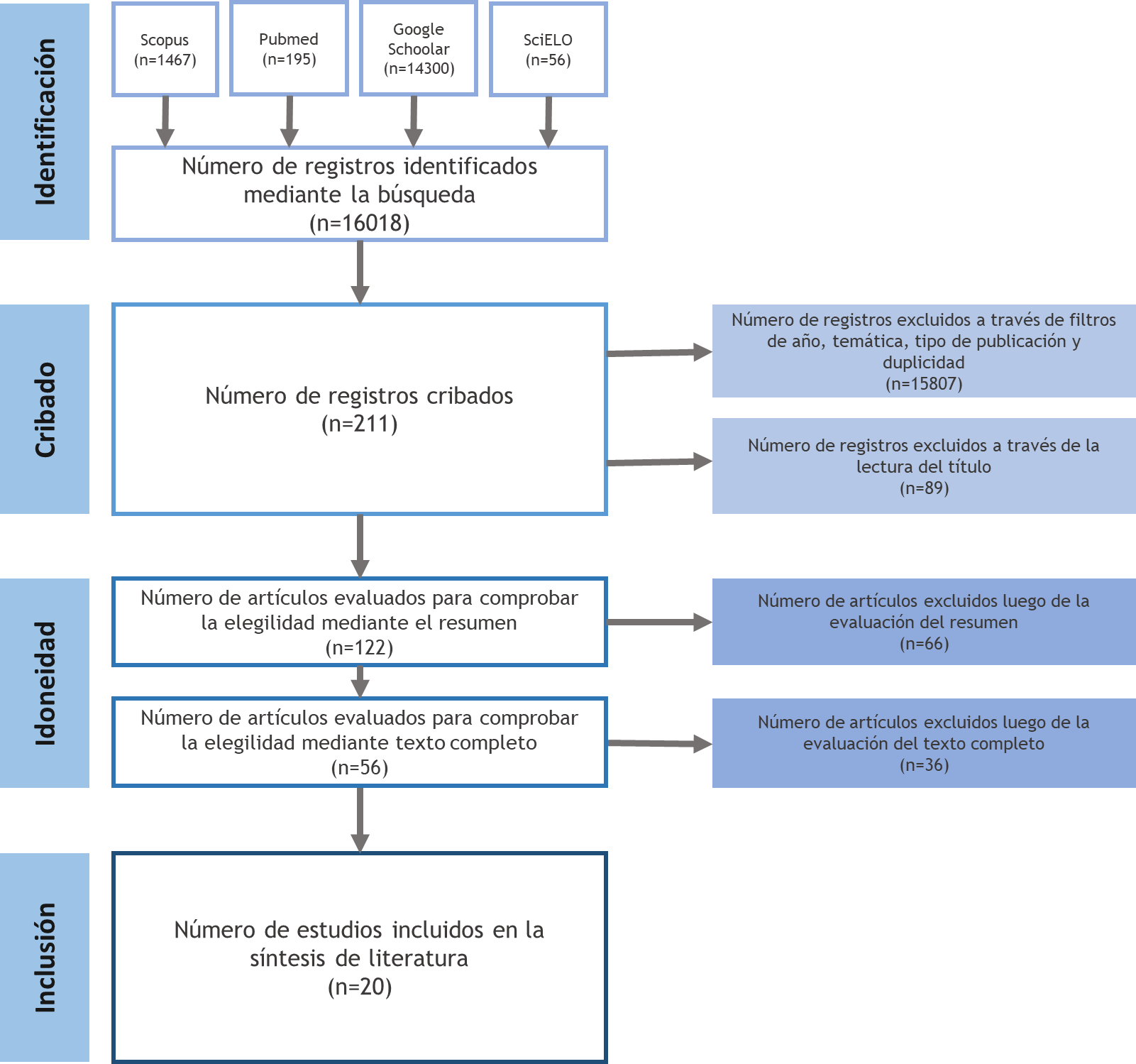
Figure 1. Flowchart of the search and selection of studies according to the PRISMA methodology
Of the 20 articles in the systematic review, 30 % were from the United States, the predominant country in the study results. Germany followed this with 10,0 %. To a lesser extent, with one article, each country (5,0 %) was also present: Argentina, Austria, Bolivia, Brazil, Canada, Cuba, Spain, Iran, Ireland, United Kingdom, and Taiwan.
This observation was expected, as the United States has been a leader in promoting and adopting health interoperability standards due to the amount of resources devoted to the research and development of these standards. In addition, the U.S. government has played an essential role in promoting health interoperability and collaboration between health and academic institutions through financial incentives.(15)
Regarding the scope of the SIS implemented according to the studies reviewed, those that offered hospital or institutional coverage predominated (60,0 %). Some 10,0 % of the studies provided information on SIS that interlinked several hospitals or institutions involved in health care. Also, 10,0 % of the studies reported systems with a national scope. Another 5,0 % were studies covering a specific area or region of a country, and another 5,0 % proposed a system covering several countries. Another 10,0 % of the studies did not specify the scope of the system in question (Figure 2).
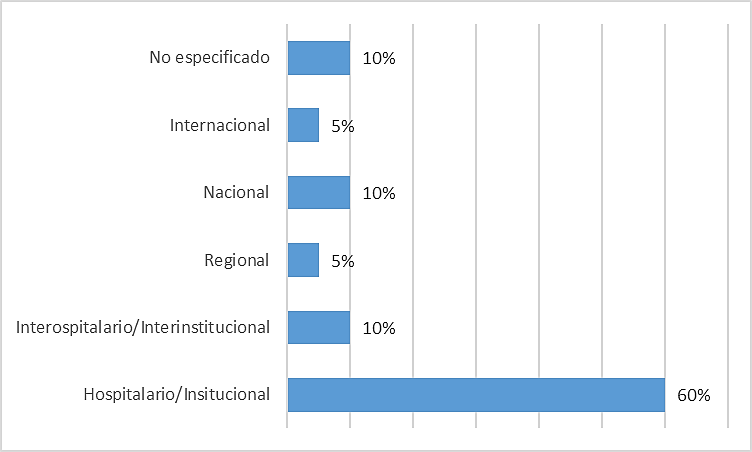
Figure 2. Number of studies according to the scope of the implemented SIS
It should be noted that the studies where the scope of the system was not specified focused on evaluating a given interoperability standard, not on the design and implementation of an SIS. These were included because they provided relevant conclusions on the results of using the standards evaluated.
The included studies showed the implementation of data and messaging, terminology, and document interoperability standards(16,17)
· Data and messaging standards refer to rules and specifications governing the structure, format, and transmission of data and messages between computer systems. This standard type is indispensable for interoperability and effective communication between systems and applications.
· Terminology standards establish the specific terminology used in a particular field or discipline. They ensure consistency and accuracy in communication and information exchange.
· Document standards define electronic documents' structure, format, and other relevant aspects. They are essential to ensure consistency, accessibility, and long-term preservation of digital documents.
At least one of these types of standards was applied in all the articles reviewed. Table 2 summarizes the standards stated in each study.
|
Table 2. Standards used in the cases analyzed |
|||
|
Study
|
Data and messaging standard |
Terminology Standard |
Document Standard |
|
Rahman Rinty et al. (2022)(18) |
HL7 |
- |
CDA |
|
Das et al. (2023)(19) |
HL7 - FHIR |
- |
CCR |
|
Kazemi-Arpanahi et al.(20) |
HL7 |
CIE-10, RxNORM, LOINC, SNOMED CT |
CDA |
|
Carvalho-Gomes et al. (2021)(21) |
- |
ISO 18104:2014 |
- |
|
Frid et al. (2022)(22) |
HL7 - FHIR |
SNOMED CT |
EN/ISO 13606 |
|
González et al. (2021)(23) |
HL7 |
CIE-10, CIE-9, NANDA, LOINC |
CDA |
|
Salvatelli et al. (2020)(24) |
HL7 , DICOM |
- |
- |
|
Choquetarqui-Guarachi (2020)(25) |
HL7 - FHIR, DICOM |
- |
- |
|
Eapen et al. (2019)(26) |
FHIR |
- |
CDM |
|
Lanyi et al. (2023)(27) |
CDS Hook, FHIR |
ATC |
- |
|
Stream et al. (2020)(28) |
- |
LOINC |
- |
|
Margheri et al. (2020)(29) |
HL7 - FHIR, DICOM |
- |
CDA |
|
Zong et al. (2021)(30) |
HL7 - FHIR |
- |
- |
|
Prud'hommeaux et al. (2021)(31) |
FHIR RDF |
- |
- |
|
Gulden et al. (2021)(32) |
HL7 - FHIR |
- |
- |
|
Gruendner et al. (2021)(33) |
FHIR |
- |
- |
|
Lee et al. (2020)(34) |
FHIR |
- |
- |
|
Plaff et al. (2019)(35) |
HL7 - FHIR |
- |
CDM |
|
Odigie et al. (2019)(36) |
FHIR, CQL |
SNOMED CT |
- |
|
Zhang et al. (2019)(37) |
FHIR |
LOINC, HPO |
- |
Leyenda
HL7: Health Level Seven
FHIR: Fast Healthcare Interoperability Resources
DICOM: Digital Imaging and Communication in Medicine
CDS Hook: Clinical Decision Support - Hook
RDF: Resource Description Framework
CQL: Contextual Query Language
ICD: International Classification of Diseases
RxNorm: Standardized Medical Prescription
LOINC: Logical Observation Identifiers Names and Codes
SNOMED CT: Systematized Nomenclature of Medicine – Clinical Terms
ISO 18104:2014: Categorical structures for the representation of nursing diagnoses and nursing actions in terminology systems.
ATC: Anatomical, Therapeutic, Chemical Classification System.
HPO: Human Phenotype Ontology Standard
NANDA: North American Nursing Diagnosis Association Standard
CDA: Clinical Document Architecture
CDM: Common Data Model
CCR: Continuity of Care Record
EN/ISO: European Norm/International Organization for Standardization
The messaging and data standards were declared in 90,0 % of the studies analyzed. Terminology and document standards were less frequent (40 %). In the messaging and data category, the HL7 - FHIR standard predominated (35,0 %). Among the terminology standards, the LOINC standard was most frequent (20,0 %), and in the document category, the CDA was most frequently observed (20,0 %) (Figure 3).
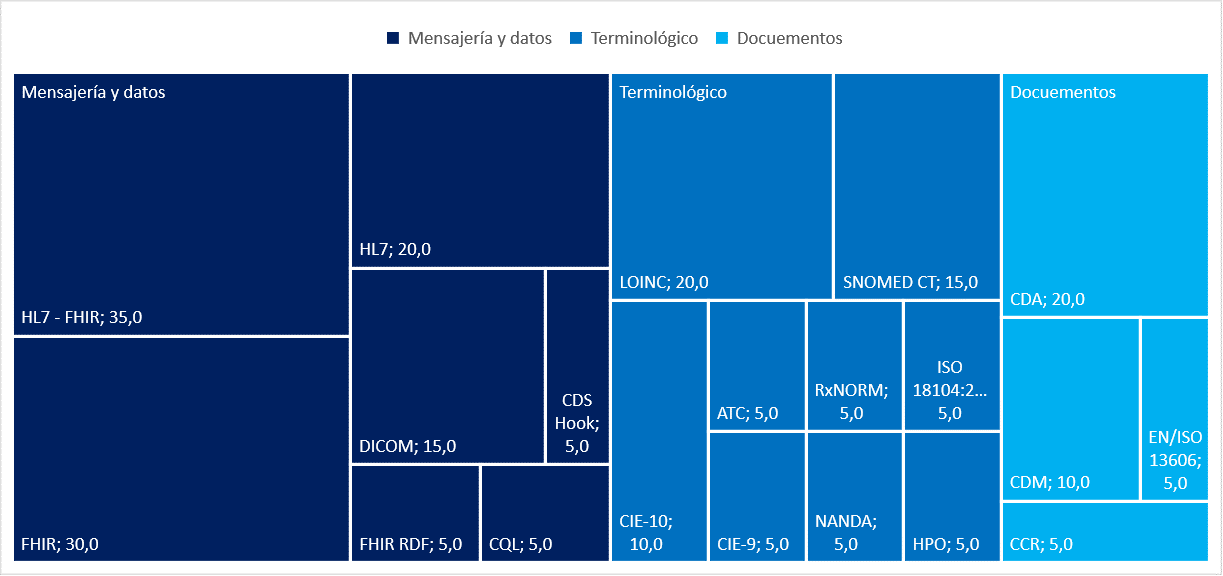
Figure 3. Distribution of the standards used in the studies analyzed
The predominance of HL7-HIR is explained by the facilities it offers for communication between different healthcare systems, which facilitates the exchange of clinical information and coordination of care. It is designed to be flexible and adaptable to different healthcare scenarios (clinical, administrative, and financial). (18,23,29,31)
LOINC and SNOMED CT are among the most popular coding standards in the health information technology industry, specifically in medical testing. LOINC codes the test method, while SNOMED CT codes non-numeric responses. The joint use of SNOMED CT and LOINC can provide a cost-effective and efficient solution for healthcare data integration. (38)
|
Tabla 3. Description of the results achieved in the application of standards by the information systems in the cases analyzed |
|||
|
Study |
Country |
Type of system |
Results and conclusions |
|
Rahman Rinty et al. (2022)(18) |
Bangladesh |
e-Health System
|
The contribution of this study is the use of the HL7 standard and message passing interface (MPI) based data retrieval in building a distributed and loosely coupled e-health system, which is revolutionary in developing countries. It is concluded that the established system helps advance e-health in developing nations worldwide. |
|
Das et al. (2023)(19) |
Irlanda |
Healthcare knowledge graph generation system
|
The article presents an approach to address the interoperability challenge in healthcare by adapting existing models and techniques. It uses a standard standardized semantic data model to generate an interconnected knowledge graph and highlights the importance of graph technologies to perform complex queries more efficiently. It also suggests the need to distinguish between different attributes at the schema level. It concludes with identifying opportunities to improve current work processes and planning for the next development phase. |
|
Kazemi-Arpanahi et al.(20) |
Irán |
Electrophysiology study report exchange information system.
|
The study presents a practical model for the presentation of the protocol for information exchange on invasive electrophysiological study of the heart based on HL7. This study identified a significant limitation: the need for comprehensive and systematic information exchange infrastructure in the country's health system, which did not allow the evaluation of the implementation of the proposed protocol. |
|
Carvalho-Gomes et al. (2021)(21) |
Brasil |
Diagnostic system and nursing interventions |
A model is presented that can be used in the electronic medical record to document nursing diagnoses and interventions in different contexts of action, from generic statements to more specific ones. |
|
Frid et al. (2022)(22) |
España |
Electronic medical record system integrated with mobile devices |
A novel methodology is described for the successful communication of standardized EHR extracts from a mobile patient application with an ontology-based clinical repository linked to an EHR. It allows the integration of data from different sources into SISs for use at different levels of care. It is considered the first study of its kind. |
|
González et al. (2021)(23) |
Cuba |
Resource planning system
|
Twelve integration points were identified between the XAVIA HIS system and the Enterprise Resource Planning (ERP) systems, which ensure integration between these systems for the first phase, including medical records, appointment centers, outpatient, emergency, laboratory, and medical programs. Messages were defined and implemented for each integration point, ensuring the exchange of information between the systems. HL7 messaging ensures the standard exchange of information between health information systems and resource planning systems. |
|
Salvatelli et al. (2020)(24) |
Argentina |
Information system for retinopathy of prematurity
|
The paper proposes the development of an ophthalmologic information system focused on the problem of retinopathies in children. The article describes the needs and motivations that justify its development, the criteria for the design of an information system of low cost and distributed characteristics that allow its use in telemedicine, and the details of a procedure for the registration of this type of images currently used in the clinic by a group of experts, which represents the starting point for the definition of the system. |
|
Choquetarqui-Guarachi (2020)(25) |
Bolivia |
Imaging information system
|
The implementation of interoperability standards in the article focuses on integrating FHIR resources with the system to standardize the X-Ray report and distribute the information. In addition, the integration with the X-ray imaging server, PACS, WADO, and HIS through REST API services is reviewed. The modeling and selection of the interoperability standard are vital variables that influence the implementation characteristics to obtain satisfactory results. The implementation of interoperability standards allowed the integration of different systems and resources to improve the management of processes and the distribution of clinical information. |
|
Eapen et al. (2019)(26) |
Canadá |
Medical Forms Management System
|
A pragmatic framework for end-to-end management of electronic products in healthcare is proposed. The framework leverages existing open-source software and standards to achieve a cost-effective and efficient solution for creating, maintaining, and sharing forms. The data collected through this framework ensures semantic aggregation and multi-level sharing. Work on the rendering engine is still in progress, and we are seeking guidance on this topic from the open-source community. |
|
Lanyi et al. (2023)(27) |
Austria |
Terminology server for clinical use |
Using FHIR and CDS are excellent additions to the digital medical environment. CDS Hooks are easy to implement and are supported by the SMART standard for easy integration into EHR systems. |
|
Stream et al. (2020)(28) |
Estados Unidos |
Not specified
|
The study concluded that real-world implementation of the LOINC code in a spectrum of laboratory settings should raise concerns about the reliability and utility of using LOINC for clinical research or to aggregate data. The study revealed a significant rate of incorrect LOINC code selection, raising concerns about the reliability and utility of LOINC use. |
|
Margheri et al. (2020)(29) |
Reino Unido |
Decentralized clinical data system
|
A Blockchain-based system is proposed to manage the provenance of healthcare documents that can seamlessly integrate with existing EHR implementations. Integration with resource-based FHIR allows operators to use the system for multiple purposes, including data reconciliation between organizations and patient consent. |
|
Zong et al. (2021)(30) |
Estados Unidos |
Data capture system for colorectal cancer patient clinical trials |
Case report forms can be considered a surrogate for representing the information needs for different types of cancers. Exploiting information needs can serve as a valuable resource to extend existing standards and ensure that case report forms can comprehensively represent relevant clinical data without losing granularity. |
|
Prud'hommeaux et al. (2021)(31) |
Estados Unidos |
Not specified
|
Developing and evaluating a Java-based toolkit for transforming and validating FHIR RDF data, one of the standardized data formats in the HL7 - FHIR specification, is described. It is concluded that FHIR RDF integrates FHIR and non-FHIR data in the healthcare and research domain. The toolkit revealed several errors in the FHIR R4 and R5 examples, demonstrating its usefulness in improving the quality of FHIR RDF data and the evolution of the FHIR specification. |
|
Gulden et al. (2021)(32) |
Alemania |
Clinical trial information system |
The results show that FHIR resources establish a harmonized view of study information from heterogeneous sources. By enabling automated data exchange between trial sites and central study registries. |
|
Gruendner et al. (2021)(33) |
Alemania |
Decentralized clinical data system
|
The study aims to show how data in FHIR format can perform statistical analysis using a preprocessing and filtering service integrated with a PSQL database. The preprocessing service was integrated with the PSQL database and the KETOS web analysis platform, allowing the generation of subsets of data with advanced medical criteria and performing statistical analysis. The study concludes that using the PSQL database to store FHIR data and the preprocessing and analysis service is feasible and beneficial for medical research. |
|
Lee et al. (2020)(34) |
Taiwán |
International clinical data management and exchange system
|
The use of interoperability standards such as FHIR and Blockchain enables the effective exchange of PHRs internationally and the security and management of PHR data. The study authors partnered with AeHIN, a regional network of digital health experts, to implement and promote the PHR platform based on these standards. The platform could also facilitate precision and individual medicine by providing a reliable and analyzable data source. |
|
Plaff et al. (2019)(35) |
Estados Unidos |
Clinical asset mapping system
|
CAMP FHIR may be an alternative to implementing new CDMs project-by-project. In addition, using FHIR as a CDM could support rare data-sharing opportunities, such as collaborations between academic medical centers and community hospitals. The adoption and use of CAMP FHIR are anticipated to foster clinical data sharing between institutions for downstream applications in translational research. |
|
Odigie et al. (2019)(36) |
Estados Unidos |
Terminology system
|
Clinical evidence logic statements were represented as sharable clinical decision support (CDS) knowledge artifacts using existing standards, SNOMED CT, FHIR, and CQL, to promote and accelerate the adoption of evidence-based practice. Limitations to standardization persist, which could be minimized with an additional set of standard terms and value sets and by adding time frames to the CQL framework. |
|
Zhang et al. (2019)(37) |
Estados Unidos |
Laboratory Test Mapping Information System
|
Electronic Health Record systems typically define laboratory test results using Laboratory Observation Names and Identification Codes and can transmit them using the Health Rapid Interoperability Resource standards. The medical implications of 2923 commonly used laboratory tests are annotated with HPO terms. Using these annotations, the designed system evaluates laboratory test results and converts each result into an HPO term. Finally, a freely available SMART on FHIR application that can be used within EHR systems is provided. |
60,0 % of the studies evaluated implemented SIS focused on clinical data management for patient care.(18,22,24,26,29,30,33,34) This result, combined with the predominance of systems at the hospital level, reaffirms the emphasis of the SIS on improving direct patient care.
The focus of 15,0 % of the studies was on systems to improve the exchange of information about the results of diagnostic tests and 10,0 % on information management for administration and resource management. The common elements of these studies include the search for solutions to improve the interoperability, efficiency, and quality of health information management, reflecting a global approach to innovation and continuous improvement in this field.(19,21,23,25,37)
These studies share common elements related to the implementation of interoperability standards, mainly HL7 and FHIR, the integration of health information systems, the importance of standardized semantics, the need to improve efficiency in clinical data management, and the application of innovative technologies for health data management.
One of the main limitations of interoperability standards is the need for complete standardization. Despite efforts to use generalized standards such as HL7, SNOMED CT, or FHIR, limitations to standardization persist, leading to the adoption of several of these standards at times. These limitations could be minimized with an additional set of standard terms and value sets and by adding time frames to the CQL framework.(36)
The contribution of these studies is significant, as they address critical challenges in system interoperability and efficiency in clinical data management in diverse settings, from developing countries(18,20,20,23,25) to more technologically advanced environments.(26,27,28,29,30,31,32,33,35,36,37) These studies reflect a common approach to innovation and continuous improvement in healthcare.
This technological and economic gap also poses an interoperability challenge. The lack of systematic information exchange infrastructure in the country's health system hinders the SIS computerization strategy.(20)
However, the association of the reviewed studies reveals a wide adoption of interoperability standards in healthcare systems and a global interest in these standards, emphasizing hospital and institutional environments. The diversity of standards used and the results obtained demonstrate the importance and significant impact of implementing interoperability standards in improving the quality and efficiency of health information systems.
CONCLUSIONS
The systematic review of literature on interoperability standards in Health Information Systems (HIS) reveals that implementing data and messaging, terminology, and document interoperability standards is fundamental to guarantee integration between the components that make up the systems, contributing to operational efficiency and information security.
Interoperability has its challenges, which are still under debate. However, implementing information technologies and standards that guarantee interoperability in the SIS has proven highly feasible. It is a way to improve the quality of care from the patient's perspective.
REFERENCES
1. Organización Panamericana de la Salud OPS. Sistemas de información y plataformas para la salud (IS). 2000. https://www.paho.org/hq/index.php?option=com_content&view=article&id=4564:information-systems-and-platforms-for-healthis&Itemid=72436&lang=es#:~:text=Sistemas%20de%20informaci%C3
2. OPS. COVID-19 y la importancia de fortalecer los Sistemas de Información. Reporte No.6. Washington DC: Departamento de Evidencia e Inteligencia para la acción en salud, Oficina del Subdirector, Organización Panamericana de la Salud; 2020. https://iris.paho.org/bitstream/handle/10665.2/52128/COVID-19FactsheetIS4H_spa.pdf?sequence=14.
3. Escobar Castellanos B, Cid-Henriquez P. Nursing care and ethical issues surgen from the technological health advance. Acta Bioeth. 2018;24(1):39–46. https://scielo.conicyt.cl/scielo.php?script=sci_arttext&pid=S1726-
4. Preciado-Rodríguez AJ, Valles-Coral MA, Lévano-Rodríguez D. Importancia del uso de sistemas de información en la automatización de historiales clínicos, una revisión sistemática. Revista Cubana de Informática Médica. 2021;13(1):e417. https://revinformatica.sld.cu/index.php/rcim/article/view/417
5. Mendoza-Ramos A. Análisis del alcance en la implementación del Sistema de Información de Salud en Colombia. Córdoba: Universidad de Córdoba; 2020.
6. Pérez-Santonja T, Gómez-Paredes L, Álvarez-Montero S, Cabello-Ballesteros L, Mombiela-Muruzabal MT. Historia clínica electrónica: evolución de la relación médicopaciente en la consulta de Atención Primaria. Semergen. 2017;43(3):175–81. https://www.sciencedirect.com/science/article/abs/pii/S1138359316300120
7. Alsalman D, Alumran A, Alrayes S, Althumairi A, Almubarak S, Alrawiai S, et al. Implementation status of health information systems in hospitals in the eastern province of Saudi Arabia. Informatics in Medicine Unlocked. 2021;22:e100499. https://doi.org/10.1016/j.imu.2020.100499
8. Institute of Electrical and Electronics Engineering – IEEE. The Authoritative Dictionary of IEEE Standards Terms, Seventh Edition. IEEE. 2000. https://doi.org/10.1109/IEEESTD.2000.322230
9. IEEE Advancing Technology for Humanity. Interoperability. EITBOK. 2017. http://eitbokwiki.org/Interoperability
10. Leal G, Guédria W, Panetto H. Interoperability assessment: A systematic literature review. Computers in Industry. 2019;106:111-132. https://doi.org/10.1016/j.compind.2019.01.002
11. Mejía GR, Cruz O, Meléndez E. Nivel de interoperabilidad en el sistema de información de la universidad pública en México. Revista de Ciencias Sociales (Ve). 2022;XXVIII(2), 56-73.
12. Felipe-Gómez L. Interoperabilidad en los Sistemas de Información Documental (SID): la información debe fluir. Códice. 2007;3(1):23-39.
13. Pagea MJ, McKenziea JE, Bossuytb PM, Boutronc I, Hoffmannd TC, Mulrowe CD, et al. Declaración PRISMA 2020: una guía actualizada para la publicación de revisiones sistemáticas. Rev Esp Cardiol. 2021;74(9):790–799 https://doi.org/10.1016/j.recesp.2021.06.0
14. Pigott TD, Polanin JR. Methodological guidance paper: High-quality meta-analysis in a systematic review. Review of Educational Research. 2020;90(1):24-46. https://doi.org/10.3102/0034654319877153
15. Mandell BF. Programa de Incentivos para la Adopción de Registros Médicos Electrónicos. Merck Manuals. 2021. https://www.merckmanuals.com/es-us/professional/temas-especiales/toma-de-decisiones-m%C3%A9dicas/registros-de-salud-electr%C3%B3nicos-y-apoyo-a-la-toma-de-decisiones-cl%C3%ADnicas
16. Gobierno de España. Ministerio de Hacienda y Administración Públicas. Catálogo de estándares. Guía de aplicación de la Norma Técnica de Interoperabilidad. 2012. https://administracionelectronica.gob.es/pae_Home/dam/jcr:97912041-c1c3-47c4-b517df9f51db321d/Guia_aplicacion_Norma_Tecnica_Interoperabilidad_Catalogo_de_estandares.pdf
17. Hospital Italiano de Buenos Aires. Addendum Interoperabilidad y Estandares. Curso Universitario de Sistemas de Información en los Sistemas de Salud. 2016. https://docplayer.es/21210626-Sistemas-de-salud-addendum-interoperabilidad-y-estandares.html
18. Rahman-Rinty M, Kumar-Prodhan U, Mijanur-Rahman M. A prospective interoperable distributed e-Health system with loose coupling in improving healthcare services for developing countries. Array. 2022;13. https://doi.org/10.1016/j.array.2021.100114
19. Das S, Hussey P. HL7-FHIR-Based ContSys Formal Ontology for Enabling Continuity of Care Data Interoperability. J. Pers. Med. 2023, 13, 1024. https://doi.org/10.3390/jpm13071024
20. Kazemi-Arpanahi H, Shanbehzadeh M, Mirbagheri E, Baradaran A. Data integration in cardiac electrophysiology ablation toward achieving proper interoperability in health information systems. J Edu Health Promot 2020;9:262. https://doi.org/10.4103/jehp.jehp_751_19
21. Carvalho-Gomes D, Abreu N, Sousa P, Moro C, Ribeiro-Carvalho D, Cubas MR. Representation of Diagnosis and Nursing Interventions in OpenEHR Archetypes. Appl Clin Inform 2021;12:340–347. https://doi.org/10.1055/s-0041-1728706.
22. Frid S, Fuentes-Expósito MA, Grau-Corral I, Amat-Fernandez C, Muñoz-Mateu M, Pastor-Duran X. Successful Integration of EN/ISO 13606–Standardized Extracts From a Patient Mobile App Into an Electronic Health Record: Description of a Methodology. JMIR Med Inform. 2022;10(10):e40344. https://medinform.jmir.org/2022/10/e40344
23. González LB, Vega IL, Rodríguez RJ, et al. Módulo ''Programas Médicos'' para el Sistema de Información Hospitalaria XAVIA HIS. Revista Cubana de Informática Médica. 2021;13(1). https://revinformatica.sld.cu/index.php/rcim/article/view/448
24. Salvatelli A, Hadad A, Evin D, Bizai G, Franseschini B, Drozdowicz B. Análisis de un Sistema de Información para Retinopatías del Prematuro (ROP). Revista Argentina De Bioingeniería. 2020;24(3):25-30. https://ri.conicet.gov.ar/handle/11336/133552
25. Choquetarqui- Guarachi R. Implementación de estándares HL7 para la interoperabilidad de aplicaciones de salud y equipos imagenológicos de Rayos X. Revista PGI. Investigación, Ciencia y Tecnología en Informática. 2020;8:160-164. https://ojs.umsa.bo/ojs/index.php/inf_fcpn_pgi/article/view/76
26. Eapen BR, Costa A, Archer N, Sartipi K. FHIRForm: An Open-Source Framework for the Management of Electronic Forms in Healthcare. Stud Health Technol Inform. 2019;257:80-85. https://pubmed.ncbi.nlm.nih.gov/30741177/
27. Lanyi L, Rinner C. ELGA Terminology Server for Clinical Decision Support: A Case-Study Using an Existing Knowledge Base, CDS Hooks and FHIR. Stud Health Technol Inform. 2023 May 2;301:125-130. https://ebooks.iospress.nl/doi/10.3233/SHTI230025
28. Stram M, Seheult J, Sinard JH, Campbell WS, Carter AB, de Baca ME, Quinn AM, Luu HS; Members of the Informatics Committee, College of American Pathologists. A Survey of LOINC Code Selection Practices Among Participants of the College of American Pathologists Coagulation (CGL) and Cardiac Markers (CRT) Proficiency Testing Programs. Arch Pathol Lab Med. 2020 May;144(5):586-596. https://doi.org/10.5858/arpa.2019-0276-oa
29. Margheri A, Masi M, Miladi A, Sassone V, Rosenzweig J. Decentralised provenance for healthcare data. International Journal of Medical Informatics. 2020;141:e104197. https://doi.org/10.1016/j.ijmedinf.2020.104197
30. Zong N, Stone DJ, Sharma DK, Wen A, Wang C, Yu Y, Huang M, Liu S, Liu H, Shi Q, Jiang G. Modeling cancer clinical trials using HL7 FHIR to support downstream applications: A case study with colorectal cancer data. Int J Med Inform. 2021 Jan;145:104308. https://linkinghub.elsevier.com/retrieve/pii/S1386505620309990
31. Prud'hommeaux E, Collins J, Booth D, Peterson KJ, Solbrig HR, Jiang G. Development of a FHIR RDF data transformation and validation framework and its evaluation. J Biomed Inform. 2021 May;117:103755. https://doi.org/10.1016/j.jbi.2021.103755.
32. Gulden C, Blasini R, Nassirian A, Stein A, Altun FB, Kirchner M, Prokosch HU, Boeker M. Prototypical Clinical Trial Registry Based on Fast Healthcare Interoperability Resources (FHIR): Design and Implementation Study. JMIR Med Inform. 2021 Jan 12;9(1):e20470. https://doi.org/10.2196/20470
33. Gruendner J, Gulden C, Kampf M, Mate S, Prokosch HU, Zierk J. A Framework for Criteria-Based Selection and Processing of Fast Healthcare Interoperability Resources (FHIR) Data for Statistical Analysis: Design and Implementation Study. JMIR Med Inform. 2021 Apr 1;9(4):e25645. https://doi.org/10.2196/25645
34. Lee HA, Kung HH, Udayasankaran JG, Kijsanayotin B, B Marcelo A, Chao LR, Hsu CY. An Architecture and Management Platform for Blockchain-Based Personal Health Record Exchange: Development and Usability Study. J Med Internet Res. 2020 Jun 9;22(6):e16748. https://doi.org/10.2196/16748
35. Pfaff ER, Champion J, Bradford RL, Clark M, Xu H, Fecho K, Krishnamurthy A, Cox S, Chute CG, Overby Taylor C, Ahalt S. Fast Healthcare Interoperability Resources (FHIR) as a Meta Model to Integrate Common Data Models: Development of a Tool and Quantitative Validation Study. JMIR Med Inform. 2019 Oct 16;7(4):e15199. https://doi.org/10.2196/15199.
36. Odigie E, Lacson R, Raja A, Osterbur D, Ip I, Schneider L, Khorasani R. Fast Healthcare Interoperability Resources, Clinical Quality Language, and Systematized Nomenclature of Medicine-Clinical Terms in Representing Clinical Evidence Logic Statements for the Use of Imaging Procedures: Descriptive Study. JMIR Med Inform. 2019 May 13;7(2):e13590. https://doi.org/10.2196/13590.
37. Zhang XA, Yates A, Vasilevsky N, Gourdine JP, Callahan TJ, Carmody LC, et al. Semantic integration of clinical laboratory tests from electronic health records for deep phenotyping and biomarker discovery. NPJ Digit Med. 2019;2:32. https://doi.org/10.1038/s41746-019-0110-4.
38. LOINC and SNOMED coding - What does it all mean? – Clinisys. 2015. https://www.clinisys.com/us/en/insight/loinc-and-snomed-coding-what-does-it-all-mean/
FINANCING
No external financing.
CONFLICT OF INTEREST
None.
AUTHORSHIP CONTRIBUTION
Conceptualization: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Data curation: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Formal analysis: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Acquisition of funds: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Research: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Methodology: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Project Administration: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Resources: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Software: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Supervision: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Validation: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Visualization: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Drafting - original draft: Carlos Canova-Barrios, Felipe Machuca-Contreras.
Writing - proofreading and editing: Carlos Canova-Barrios, Felipe Machuca-Contreras.