doi: 10.56294/mw2023100
ORIGINAL
Adaptive Control Strategies for Bioprinting Tissues and Organs in Biomedical Engineering Applications
Estrategias de control adaptativo para la bioimpresión de tejidos y órganos en aplicaciones de ingeniería biomédica
Chandan Das1 ![]() *, Renuka
Jyothi S2
*, Renuka
Jyothi S2 ![]() , Rohini3
, Rohini3 ![]()
1IMS and SUM Hospital, Siksha ‘O’ Anusandhan (deemed to be University), Department of General Medicine. Bhubaneswar, Odisha, India.
2JAIN (Deemed-to-be University), Department of Biotechnology and Genetics. Bangalore, Karnataka, India.
3Noida International University, Department of Anaesthesia. Greater Noida, Uttar Pradesh, India.
Cite as: Das C, S RJ, Rohini. Adaptive Control Strategies for Bioprinting Tissues and Organs in Biomedical Engineering Applications. Seminars in Medical Writing and Education. 2023; 2:100. https://doi.org/10.56294/mw2023100
Submitted: 03-09-2022 Revised: 15-12-2022 Accepted: 22-02-2023 Published: 23-02-2023
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding Author: Chandan Das *
ABSTRACT
Bioprinting is a revolutionary technology in the area of producing organs and tissues in biomedical engineering. Despite potential, variability in bioinks, nonlinear dynamics, and variability in the environment cause precision and reliability in the process. The current methods, such as the Proportional-Integral-Derivative (PID) controller, are plagued with disadvantages such as slow convergence, susceptibility to local minima, and a lack of adaptability in variable conditions. Overcoming these disadvantages, a hybrid system is presented, Scalable Shuffled Shepherd-tuned adaptive PID controller (SSS-Adaptive PID). The Adaptive PID Controller dynamically adapts in real time with variable conditions and guarantees stability and responsiveness. The SSS Optimization maximizes optimization with increased convergence and enhanced robustness and overcomes the issues with time-varying and nonlinear conditions in bioprinting. The results of the experiments are reduced Rise Time (0,21 × 10⁻5 sec), Settling Time (0,05 × 10⁻3 sec) and peak time (0,09 × 10⁻3 sec). Improved cell viability and structure fidelity are also noted in printed constructs. It overcomes the disadvantages to conventional methods and is a consistent and efficient solution in 3D bioprinting. By permitting adaptive and accurate control, the system makes high-quality organs and tissues production possible in biomedical applications.
Keywords: Bioprinting Tissues; Biomedical Engineering; Adaptive Control Strategies; Scalable Shuffled Shepherd-Tuned Adaptive PID Controller (SSS-Adaptive PID).
RESUMEN
La bioimpresión es una tecnología revolucionaria producción de órganos y tejidos en ingeniería biomédica. A pesar del potencial de potencial, la variabilidad de las biotintas, la dinámica no lineal y la variabilidad del entorno provocan imprecisión y fiabilidad en el proceso. Los actuales métodos actuales, como el controlador Proporcional-Integral-Derivativo (PID), están tienen desventajas como la lentitud de convergencia, la propensión a los mínimos locales y la falta de adaptación. y la falta de adaptabilidad en condiciones variables. Para superar estos desventajas, se presenta un sistema híbrido, Scalable Shuffled Shepherd-tuned adaptativo (SSS-Adaptive PID). El controlador PID adaptativo se adapta dinámicamente en tiempo real con condiciones variables y garantiza estabilidad y capacidad de respuesta. La optimización SSS maximiza la optimización con mayor convergencia y robustez, y supera los problemas que plantean las condiciones condiciones variables en el tiempo y no lineales en bioimpresión. Los resultados de los experimentos son la reducción del tiempo de subida (0,21 × 10-5 seg), el tiempo de asentamiento (0,05 × 10-3 seg) y el tiempo de pico (0,09 × 10-3 seg). También se observa una mejora de la viabilidad celular y de la fidelidad de la estructura en las construcciones impresas impresas. Supera los inconvenientes de los métodos convencionales y es una solución coherente y eficaz para la bioimpresión 3D. Al permitir un control adaptable y de órganos y tejidos de alta calidad en aplicaciones biomédicas. en aplicaciones biomédicas.
Palabras clave: Bioprinting Tissues; Biomedical Engineering; Adaptive Control Adaptativo; Controlador PID Adaptativo Scalable Shuffled Shepherd-Tuned (SSS-Adaptive PID).
INTRODUCTION
One of this century’s most cutting-edge and inventive technologies, bioprinting has gained international attention and transformed a number of industries, including tissue engineering, regenerative medicine, and the pharmaceutical sector. It describes the process of printing different biomaterials with integrated living cells using three-dimensional (3D) printing technology. Biomaterials and polymers are methodically layered in a predetermined stacking sequence for the bioprinting of cells, tissues, and organs.(1) To assist the reader in navigating the complexity of the numerous tissues and organs, the three germ layers, ectoderm, endoderm, and mesoderm, from which all organs are generated, are briefly explained here. Discuss the gastrulation—the spatiotemporal arrangement and cellular association of these layers—and highlight their enormous difficulty, which is still not completely understood.(2)
The advantages of 3D bioprinting over standard 3D printing are numerous and include cost-effectiveness, scalability, high-resolution cell deposition, and accurate cell dispersion.(3) By leveraging 3D printing techniques and bioinks composed of living cells and biomaterials, bioprinting offers significant potential for regenerative medicine, tissue engineering, and pharmaceutical research.(4) These restrictions lead to inconsistencies in bioink deposition, structural deformations, and decreased cell viability, lowering the quality and performance of printed tissues.(5)
Traditional control systems, such as the Proportional-Integral-Derivative (PID) controller, have long been utilized to govern printing parameters and assure reliable production.(6) However, typical PID controllers frequently exhibit delayed convergence, vulnerability to local minima, and an inability to properly adapt to the dynamic conditions of the bioprinting process. These limits need the creation of more robust and adaptable control systems to improve the precision and reproducibility of bioprinted structures.(7)
The integration of deep learning (DL) approaches into 3D bioprinting was examined to improve precision, efficiency, and the maturation of tissues.(8) DL techniques were applied to several stages of the bioprinting process, including post-printing tissue maturation, printing parameter optimization, image processing, segmentation, and in-situ mistake correction. According to the findings, DL greatly improved real-time error correction, printing parameter optimization, and image correctness, all of which increased the structural and functional integrity of printing tissue. Alginate hydrogel’s benefits and drawbacks as a bioink for 3D bioprinting were examined.(9) To solve problems including limited printability, uneven biodegradability, and poor cell adhesion, a variety of material changes and biofabrication techniques were examined. The results showed that although alginate hydrogel had good gelation and biocompatibility qualities, its drawbacks need sophisticated adjustments to improve performance for biomedical applications.
Applications of 3D and 4D printing technology advancements in tissue engineering, namely in patient-specific scaffold manufacturing and guided tissue regeneration, were investigated.(10) Different printing processes, material choices, and stimuli-responsive characteristics were examined and contrasted with conventional scaffold manufacturing procedures. The results demonstrated that while 4D printing added dynamic possibilities through intelligent materials, 3D printing allowed for the exact construction of complicated structures. Cardiovascular (CV) tissue engineering was investigated to overcome the limitations of current tissue replacement technologies and improve in vivo transplantation and disease modeling.(11) A variety of CV tissue-specific biomaterials, 3D bioprinting processes, and assessment approaches were examined in order to improve their anatomical and physiological relevance. The results demonstrated that while novel evaluation approaches allowed for real-time, non-invasive monitoring, modern biomaterials and bioprinting processes enhanced biocompatibility, durability, and functional integration.
The ability of intraoperative bioprinting (IOB), especially for composite and vascularized tissues, to immediately heal tissue abnormalities in surgical situations was investigated.(12) Engineering and clinical challenges, including precision, biocompatibility, and real-time defect scanning, were analyzed alongside the feasibility of translating bioprinting from research to clinical applications. The results showed that although IOB increased surgical efficiency and precision, problems with tissue integration, printing speed, and bioink composition persisted. Multiple biofabrication technologies were combined to address the limitations of single-component approaches and improve tissue functionality.(13) Advances in merging inkjet, extrusion, and light-assisted printing were investigated, as well as the incorporation of additive manufacturing (AM), digital design, and artificial intelligence (AI). The outcomes show that the structural and functional characteristics of engineered tissue were optimized by the application of digital design and artificial intelligence to enable biofabrication operations. With all these processes integration, biomimetic tissue engineering was optimized even further.
The guidelines, legal frameworks, and challenges related to the use of tissue-engineered pharmaceutical products (TEMPs) and 3D bioprinted tissues in clinical contexts were examined.(14) Approval process, manufacturing standards, and laws have been examined with a focus on the complexities of materials and bioprinting methods. Together, the results suggest that while 3D bioprinted tissues and tissue-engineered medical products (TEMPs) represented potential for therapy, there were still numerous challenges to be solved, particularly with regard to the requirement for standardized procedures and regulatory limitations. Developments in cardiac tissue engineering (CTE) using 3D bioprinting were investigated in order to improve heart repair and treat myocardial insufficiency.(15) By examining different bioink formulations and applications, such as heart patches and modified cardiac muscle, conventional techniques and 3D bioprinting were contrasted. According to the results, 3D bioprinting made it possible to create complex cardiac designs with integrated cells, which developed the likelihood of tissue restoration.
Aim: To introduce the SSS-Adaptive PID controller to address bioprinting challenges, improving precision, stability, and robustness for producing high-quality organs and tissues in biomedical applications.
METHOD
To increase the precision and reliability of 3D bioprinting, the project aims to develop an SSS-Adaptive PID controller that dynamically adjusts control settings in real time. The suggested approach overcomes issues, including nonlinear dynamics, environmental variations, and bioink variability, to increase structural fidelity and bioink deposition consistency. Stability is guaranteed by the adaptive PID controller, and convergence speed and robustness are improved by SSS optimization. Figure 1 displays the methods involved in the proposed model.

Figure 1. Overall Flow Diagram for Proposed Method
Mechanical Structure and Control System of the 3D Bioprinter
There are several different designs for 3D bioprinters, such as Cartesian, Delta, and Core XY designs. The majority of these systems have an extruder and a heated bed that are incorporated into a Cartesian coordinate system. In this research, a Cartesian-type 3D bioprinter with a study mechanical construction tuned for accuracy and dependability was created especially for the creation of artificial tissues and organs. The printer ensures excellent accuracy in layer deposition by moving along the X, Y, and Z axes with a ballscrew. The system’s entire measurements are 600 × 600 × 700 mm, and it has seven industrial-grade servo motors for precise and seamless movement.
The extruder pendulum system with three nozzles (diameters 0,2 mm, 0,4 mm, and 0,6 mm) is a special feature in the 3D bioprinter. The device improved bioink deposit in a wide array of tissue architectures through adaptive switching among nozzles. Cell viability, structure, and bioink consistency in the process are under strict multi-nozzle system command in the process of bioprinting. The introduction here is the SSS-Adaptive PID controller, a solution in overcoming conventional control method shortcomings. The conventional PID controllers are slow in reaction, vulnerable to local minima, and not adequate in terms of flexibility in dealing with nonlinear conditions in bioprinting. The proposed SSS-Adaptive PID controller ensures performance in reaction and stability in bioink variability and in environmental drift through adaptive adjustment in control in real time. Furthermore, with enhanced convergence and greater flexibility in time-varying conditions in bioprinting, efficiency in control is improved via the SSS optimization algorithm.
Besides controlling the seven-axis motion system, the SSS-Adaptive PID controller also adapts the extruder pendulum system with the ability to switch among nozzle diameters seamlessly. The method drastically improves layer precision, lowers deposition inaccuracies, and improves the reproducibility of printed constructs, and is proven in experiments. Artificial high-fidelity organs and tissues are obtainable with the improved control strategy, with potential in future dependable and efficient bioprinting in regenerative medicine and biomedical engineering.
Scalable Shuffled Shepherd-tuned adaptive PID controller (SSS-Adaptive PID)
In 3D bioprinting, system stability, precise control over deposition, and parameter adaptation in real time are facilitated by the Scalable Shuffled Shepherd-tuned adaptive PID controller. Faster convergence, improved robustness against nonlinear oscillations, and dynamic adaptation are provided by the Shuffled Shepherd Optimization (SSO). Adaptation against bioink variability and environmental conditions is maximized by the adaptive PID system through continuous fine-tuning of the control parameters. Adaptive PID combined with SSO is an efficient and reliable precision bioprinting strategy with improved precision in deposition, reduced distortion in structure, and guaranteed cell viability.
Adaptive PID Controller
The PID controller, which uses derivative (Lo), integral (Lj), and proportional (Lc) terms for system regulation, is popular because it is straightforward. However, the SSS-Adaptive PID controller, which improves stability, adaptability, and deposition accuracy, is required in 3D bioprinting due to its limits in managing nonlinear dynamics and bioink variability.
The following equation (1) and (2) are the PID controller’s Laplace transfer function:

The extruder pendulum system’s intended and actual trajectories are represented by θdes and θact in 3D bioprinting. The error (f) in the control system is defined as the difference between the actual and desired trajectories.
![]()
The 3D bioprinting extruder pendulum system’s closed-loop control system’s transfer function is provided by equation (4).
![]()
When the extruder and nozzle system’s transfer function is represented by HjD(t).
Where:
j=1 and j=2 denotes the three-nozzle configuration.
The 3D bioprinting control system’s PID parameters are adjusted iteratively to maximize efficiency.
Lv represents Lo, while Lj and Lc are initially set to zero. The final value of Lv, known as the ultimate gain (Lcr), and the ultimate oscillation period (Sv) are used to determine the PID controller parameters, ensuring stability and precision in the extruder pendulum system.
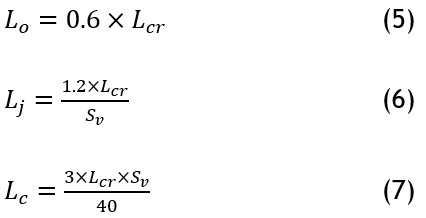
Scalable Shuffled Shepherd Optimization (SSSO)
The SSSO is presented here in order to advance the adaptive PID control system in 3D bioprinting. The process is enhanced with two significant features in order to obtain improved precision and convergence. Initialization is improved with opposition-based learning (OBL) in order to obtain a better optimum parameter search and avoid the algorithm getting trapped in local optimum. Secondly, adaptive bioink consistency in deposition and structure fidelity is provided with a statistically reconstructed step size, with increased exploration in initial iterations and avoiding premature convergence.
The OBL technique is used to improve the SSS optimization algorithm’s initialization procedure in 3D bioprinting control. The suggested approach makes use of all three of the advanced variations of OBL (opposite learning), including Super-Opposite-Based Learning (SOBL), Quasi-OBL (QOBL), and Quasi-Reflection OBL (QROBL). These methods enhance parameter optimization, guaranteeing greater flexibility and accuracy in managing the dynamics of bioink deposition and extrusion. The definition of the opposite of a given answer x is as following equation (8).
![]()
Therefore, the opposite of the provided solution is represented by a study. Different OBL variations are produced using both the original solution and its opposite, improving adaptive parameter tuning for accurate bioink deposition in 3D bioprinting. These OBL variations are produced using the following:
The Quasi-Reflection solution (QRX) for the given parameter is defined as an arbitrarily produced value between and the midpoint.
![]()
This method improves adaptive parameter adjustment in the context of 3D bioprinting, increasing system stability and the precision of bioink deposition. Equation (9) is used to regulate the QRX for the control variable.
![]()
For a given parameter, the Quasi-Opposite solution (QX) is a randomly generated number that falls between the midway and its opposite (OX). This method improves adaptive tuning of the PID parameters in the context of 3D bioprinting control, guaranteeing increased stability and accuracy in bioink deposition. The following equation (10) is used to calculate the QX.
![]()
The following equation (11) defines the Super-Opposite solution (SUX) for a given parameter w.
![]()
Every randomly generated solution and those obtained from the above computations are taken into account to guarantee that the overall residents exceed 5 × members. 4 more solutions are designed for every randomly produced solution. The top members are chosen as the starting residents for the adaptive PID tuning procedure in 3D bioprinting control after the full population has been sorted according to solution quality.
Despite being a population-based metaheuristic, the SSS optimization process has drawbacks, including the potential to become stuck in local minima close to an ideal solution because of a lack of population variety. The step size technique for creating new solutions is altered to solve this problem. A flow control strategy is presented in which the standard method is used to calculate the step size if the number that is created at random is less than 0,8. If not, the statistically adjusted step size is used to regenerate 20 % of the control variables in the chosen agent, improving adaptive PID tuning in 3D bioprinting control.
![]()
The function V generates an integer at random from an uninterrupted regular distribution, with both upper and lower bounds provided by Meani,r-Stdi,r-sigmai,r and Meani,r+Stdi,r+sigmai,r, respectively. Meani,r and Stdi,r denote the mean and standard deviation of the rth control variable in the ith group, while sigmai.r is a parameter that ensures the statistically adjusted step size functions effectively when the adaptive PID tuning process in 3D bioprinting control converges to a specific value.
![]()
Design of SSS-Adaptive PID for 3D Bioprinting
A typical PID controller is used to regulate 3D bioprinters, which are utilized to create fake tissues and organs. However, in order to decrease fabrication errors, particularly surface defects in bioprinted parts, an adaptive PID controller is suggested. In this section, a PID controller optimized using the Scalable Shuffled Shepherd-Tuned (SSS) algorithm is proposed to enhance the transient reply of the 3D bioprinter. The diagram of the adaptive PID controller based on SSS is depicted in figure 2, in which θq is the reference angular position, the closed-loop output (θ), θf is the angular position error, and vr is the controller’s control signal, is the actual angular position.

Figure 2. Design of SSS-Adaptive PID for 3D Bioprinting
RESULTS
By attaining shorter rising time, shorter settling time, lower overshooting, and improved peak time, the SSS-Adaptive PID controller was superior in major dynamic response criteria over conventional PID controllers. The adaptive tuning process provided a quick system stabilization and reduced oscillation while achieving an accurate bioink deposit. The incorporation of SSO provided improved convergence, successfully reducing the errors and increasing consistency in bioprinting.
Experimental Setup
The SSS-Adaptive PID was implemented on a Windows 10 machine with a RAM of 16GB in order to process efficiently. The system integrated Shuffled Shepherd Optimization (SSO), nonlinear dynamic compensation, and adaptive PID tuning in real-time with the use of MATLAB/Simulink.
Experimental Result
The performance of the SSS-Adaptive PID is evaluated in terms of the rise time, the settling time (2 %), the overshooting, and the peak time. The performance is also evaluated with conventional PID controllers and other adaptive controllers such as classical PID and SSS-Adaptive PID controller. The performance of each control method is evaluated in important performance criteria, and these are presented in table 1.
|
Table 1. 3D Printer Results of Transient Response Analysis |
||||
|
Controller |
Rise Time (sec) |
Settling Time (2 %) (sec) |
Overshoot (%) |
Peak Time (sec) |
|
PID |
0,78 × 10-3 |
0,97 × 10-2 |
41,67 |
0,36 × 10-2 |
|
SSS-Adaptive PID (Proposed) |
0,21 × 10-5 |
0,05 × 10-3 |
No overshoot |
0,09 × 10-3 |
Rise Time
A shorter rising time will result in more accurate and timelier bioink deposition since the system can respond more quickly. The SSS-Adaptive PID controller (0,21 × 10-5 sec), compared to the PID controller (0,78 × 10-³ sec), yields a significantly shorter rising time and is a more accurate reflection of the responsiveness of the system.
Settling Time (2 %)
A shorter settling time ensures a quicker system stabilization, decreasing bioink extrusion inaccuracies and improving consistency in prints. The amount of time taken by the system output in order not to pass through any oscillation and settle within a tolerance level of 2 % is termed. The desired state is reached with better stability and in a shorter amount of time with the SSS-Adaptive PID controller and settles much faster than the PID controller (0,97 × 10-² sec) at a rate of 0,05 × 10-3 sec.
Overshoot
Overshoot can cause significant bioink deposit distortion, resulting in uneven layer thickness and low structure quality. The removal of overshoot guarantees precision and uniform bioprinting. In bioprinting, the excessive overshoot (41,67 %) in the PID controller would result in system instability and inaccuracies. In the presence of the SSS-Adaptive PID controller, overshoot is eliminated completely, guaranteeing precision without oscillation.
Peak Time
A reduced peak ensures the system is responding in accordance with its potential without unnecessary lags, accelerating and increasing the efficiency in the process of printing. The ability of the SSS-Adaptive PID controller in achieving optimum conditions in a shorter time is proven with a substantially shorter peak response (0,09 × 10⁻3 sec) in comparison with the PID controller (0,36 × 10-2 sec).
DISCUSSION
The basis for process optimization is the way that different control techniques impact 3D bioprinting stability and accuracy. The transient response results exhibit how crucial adaptive control methods are in achieving precision and consistency in bioink deposition. The benefits of parameter adjustment in curbing oscillation in nonlinear systems are brought out in comparing the control methods. The SSS-Adaptive PID method was better than the conventional PID method in having the shortest reaction time. The capability in the presented controller in achieving steady state in a short while with precision and seamless layer deposition is brought out in the 0,21 × 10-5 sec rising time and the 0,05 × 10-3 sec settling time. In the SSS-Adaptive PID controller, there is no overshooting, and this brings out how adaptive optimization is effective in curbing system instability. The printed tissues could suffer from structure unevenness and uneven layer thickness because of the high overshooting (41,67 %) brought about by the PID method. It brings out how crucial sophisticated control systems are in cell viability and structure. Moreover, a faster transition in steady state is made possible with the decreased peak time (0,09 × 10-3 sec) in the SSS-Adaptive PID method, with reduced loss in materials and increased precision in deposition. These results exhibit how crucial the ability in real-time is in curbing variability in bioink and fluctuation in the environment. The research brings out how high-precision bioprinting is made possible with sophisticated control algorithms and coupled with hardware optimization. It is crucial in a way that ensures steady bioink flow, swift stabilization, and increased precision in prints. In consideration of the better performance in the SSS-Adaptive PID method, future bioprinting control systems would require intelligent optimization algorithms in order to boost precision in deposition, curtail loss in materials, and enable production in viable tissues and organs.
CONCLUSIONS
The effectiveness is improved with the solution of problems in bioprinting via adaptive and intelligent control systems. Research shows how Scalable Shuffled Shepherd-tuned Adaptive PID (SSS-Adaptive PID) controller improves precision and trustworthiness over conventional PID approaches whose adaptation is slow and limited. The combination of real-time adaptive control and SSS optimization improves layer precision, lowers percentages in errors, and makes bioink deposition consistent, resulting in enhanced cell viability and structure fidelity in printed constructs. These advantages highlight the imperative need in utilizing advanced control algorithms in treating nonlinear and time-varying conditions in bioprinting. The results show how conventional fixed-parameter control approaches are not sufficient in ensuring precision and stability in complex conditions in bioprinting. The adaptive approach is valuable in biomedical applications involving high-precision production in organs and tissues. Research is constrained in such a way that there is a requirement in broader validation in multiple conditions in bioprinting and bioink formulations. Future research is required in on-site monitoring tools and machine learning-based optimization in order to improve the precision and efficiency in bioprinting.
BIBLIOGRAPHIC REFERENCES
1. Aljohani W, Ullah MW, Zhang X, Yang G. Bioprinting and its applications in tissue engineering and regenerative medicine. International journal of biological macromolecules. 2018 Feb 1;107:261-75. https://doi.org/10.1016/j.ijbiomac.2017.08.171
2. Mota C, Camarero-Espinosa S, Baker MB, Wieringa P, Moroni L. Bioprinting: from tissue and organ development to in vitro models. Chemical reviews. 2020 May 14;120(19):10547-607. https://doi.org/10.1021/acs.chemrev.9b00789
3. Xie Z, Gao M, Lobo AO, Webster TJ. 3D bioprinting in tissue engineering for medical applications: the classic and the hybrid. Polymers. 2020 Jul 31;12(8):1717. https://doi.org/10.3390/polym12081717
4. Ghidini T. Regenerative medicine and 3D bioprinting for human space exploration and planet colonisation. Journal of thoracic disease. 2018 Jul;10(Suppl 20):S2363. 10.21037/jtd.2018.03.19
5. Kim BS, Das S, Jang J, Cho DW. Decellularized extracellular matrix-based bioinks for engineering tissue-and organ-specific microenvironments. Chemical reviews. 2020 Jul 31;120(19):10608-61. https://doi.org/10.1021/acs.chemrev.9b00808
6. Aboelhassan A, Abdelgeliel M, Zakzouk EE, Galea M. Design and Implementation of model predictive control based PID controller for industrial applications. Energies. 2020 Dec 14;13(24):6594. https://doi.org/10.3390/en13246594
7. Heinrich MA, Liu W, Jimenez A, Yang J, Akpek A, Liu X, Pi Q, Mu X, Hu N, Schiffelers RM, Prakash J. 3D bioprinting: from benches to translational applications. Small. 2019 Jun;15(23):1805510. https://doi.org/10.1002/smll.201805510
8. Ng WL, Chan A, Ong YS, Chua CK. Deep learning for fabrication and maturation of 3D bioprinted tissues and organs. Virtual and Physical Prototyping. 2020 Jul 2;15(3):340-58. https://doi.org/10.1080/17452759.2020.1771741
9. Gao Q, Kim BS, Gao G. Advanced strategies for 3D bioprinting of tissue and organ analogs using alginate hydrogel bioinks. Marine Drugs. 2021 Dec 15;19(12):708. https://doi.org/10.3390/md19120708
10. Tamay DG, Dursun Usal T, Alagoz AS, Yucel D, Hasirci N, Hasirci V. 3D and 4D printing of polymers for tissue engineering applications. Frontiers in bioengineering and biotechnology. 2019 Jul 9;7:164. https://doi.org/10.3389/fbioe.2019.00164
11. Yong U, Lee S, Jung S, Jang J. Interdisciplinary approaches to advanced cardiovascular tissue engineering: ECM-based biomaterials, 3D bioprinting, and its assessment. Progress in Biomedical Engineering. 2020 Sep 18;2(4):042003. DOI 10.1088/2516-1091/abb211
12. Wu Y, Ravnic DJ, Ozbolat IT. Intraoperative bioprinting: repairing tissues and organs in a surgical setting. Trends in biotechnology. 2020 Jun 1;38(6):594-605. DOI: 10.1016/j.tibtech.2020.01.004
13. Castilho M, de Ruijter M, Beirne S, Villette CC, Ito K, Wallace GG, Malda J. Multitechnology biofabrication: a new approach for the manufacturing of functional tissue structures?. Trends in biotechnology. 2020 Dec 1;38(12):1316-28. DOI: 10.1016/j.tibtech.2020.04.014
14. Sekar MP, Budharaju H, Zennifer A, Sethuraman S, Vermeulen N, Sundaramurthi D, Kalaskar DM. Current standards and ethical landscape of engineered tissues—3D bioprinting perspective. Journal of tissue engineering. 2021 Jul;12:20417314211027677. https://doi.org/10.1177/20417314211027677
15. Wang Z, Wang L, Li T, Liu S, Guo B, Huang W, Wu Y. 3D bioprinting in cardiac tissue engineering. Theranostics. 2021 Jul 6;11(16):7948. doi: 10.7150/thno.61621
FINANCING
None.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Chandan Das, Renuka Jyothi S, Rohini.
Data curation: Chandan Das, Renuka Jyothi S, Rohini.
Formal analysis: Chandan Das, Renuka Jyothi S, Rohini.
Drafting - original draft: Chandan Das, Renuka Jyothi S, Rohini.
Writing - proofreading and editing: Chandan Das, Renuka Jyothi S, Rohini.