doi: 10.56294/mw2023119
ORIGINAL
Ethical Challenges in Pharmaceutical Marketing: A Multidimensional Analysis of Healthcare Industry Practices
Retos éticos del marketing farmacéutico: Análisis multidimensional de las prácticas de la industria sanitaria
Malathi H1 ![]() *, Rohini2
*, Rohini2 ![]() , Laxmidhar Maharana3
, Laxmidhar Maharana3 ![]()
1JAIN (Deemed-to-be University), Department of Biotechnology and Genetics. Bangalore, Karnataka, India.
2Noida International University, Department of Anaesthesia, Greater Noida. Uttar Pradesh, India.
3Siksha ‘O’ Anusandhan (Deemed to be University), Department of Pharmaceutical Sciences. Bhubaneswar, Odisha, India.
Cite as: H M, Rohini, Maharana L. Ethical Challenges in Pharmaceutical Marketing: A Multidimensional Analysis of Healthcare Industry Practices. Seminars in Medical Writing and Education. 2023; 2:119. https://doi.org/10.56294/mw2023119
Submitted: 09-09-2022 Revised: 21-12-2022 Accepted: 25-02-2023 Published: 26-02-2023
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding Author: Malathi H *
ABSTRACT
Pharmaceutical marketing plays a crucial role in shaping healthcare practices, influencing both medical professionals and consumers. However, ethical concerns have emerged regarding deceptive advertising, financial incentives for healthcare providers, and the exploitation of vulnerable populations. The objective is to assess how various marketing strategies influence public and healthcare professionals’ ethical perceptions of pharmaceutical industry practices and identify areas for improvement in regulatory frameworks. Independent variables include Direct-to-Consumer Advertising (DTCA), Financial Incentives (FI) to healthcare providers, Transparency of Drug Information (TDI), Regulatory Compliance (RC), and Public Trust (PT) in the pharmaceutical industry. The dependent variable is the Ethical Perception of Pharmaceutical Marketing(EPPM),measured by the perceptions of healthcare professionals and patients on the ethical practices in pharmaceutical marketing and their trust in the industry. Data is collected through surveys of 306 healthcare professionals and 216 patients, along with interviews with pharmaceutical executives, using descriptive statistics and regression analysis in SPSS 25. Regression analysis reveals significant correlations between pharmaceutical marketing practices and public trust. Descriptive statistics were performed to identify relationships between revealed varying levels of ethical concerns, particularly related to DTCA and financial incentives. The findings suggest that increased transparency, adherence to regulations, and ethical advertising could improve industry practices and enhance public confidence in pharmaceutical companies.
Keywords: Pharmaceutical Marketing; Healthcare Industry; Ethical Challenges; Descriptive Statistics.
RESUMEN
El marketing farmacéutico desempeña un papel crucial en la configuración de las prácticas sanitarias, influyendo tanto en los profesionales médicos como en los consumidores. Sin embargo, han surgido problemas éticos relacionados con la publicidad engañosa, los incentivos económicos a los profesionales sanitarios y la explotación de poblaciones vulnerables. El objetivo es evaluar cómo influyen las distintas estrategias de marketing en las percepciones éticas del público y los profesionales sanitarios sobre las prácticas de la industria farmacéutica e identificar áreas de mejora en los marcos normativos. Las variables independientes son la publicidad directa al consumidor (PVDC), los incentivos económicos (IF) a los profesionales sanitarios, la transparencia de la información sobre medicamentos (TDI), el cumplimiento de la normativa (CR) y la confianza pública (CP) en la industria farmacéutica. La variable dependiente es la Percepción Ética del Marketing Farmacéutico (EPPM), medida por las percepciones de los profesionales sanitarios y los pacientes sobre las prácticas éticas en el marketing farmacéutico y su confianza en la industria. Los datos se recogen mediante encuestas a 306 profesionales sanitarios y 216 pacientes, junto con entrevistas a directivos farmacéuticos, utilizando estadística descriptiva y análisis de regresión en SPSS 25. El análisis de regresión revela correlaciones significativas entre las prácticas de marketing farmacéutico y la confianza del público. Se realizaron estadísticas descriptivas para identificar las relaciones entre los distintos niveles de preocupación ética revelados, especialmente en relación con la publicidad directa al consumidor y los incentivos económicos. Los resultados sugieren que el aumento de la transparencia, el cumplimiento de la normativa y la publicidad ética podrían mejorar las prácticas de la industria y aumentar la confianza del público en las empresas farmacéuticas.
Palabras clave: Marketing Farmacéutico; Industria Sanitaria; Desafíos Éticos; Estadística Descriptiva.
INTRODUCTION
The pharmaceutical sector utilizes two independent terms, marketing for pharmaceutical and medico, to support its products and names to produce significant profits and occupy a variety of marketing and medical methods.(1) The healthcare sector is working to improve access, enhance productivity, and provide superior care at a lower cost. For medical experts, chronic, severe neurological conditions have presented difficulties.(2) In the resource-intensive clinical area of healthcare, huge amounts of data are frequently generated, retrieved, and shared. It is both essential and extremely challenging to store and transmit this enormous amount of data because of its critical nature and limiting factors like security and privacy.(3) Personnel expenses are the most significant contribution to hospital expenditures, followed by purchasing and material costs. Hospitals should use automated order collecting to lower delivery frequency and assist effective inventory management, reduce storage space to enhance inventory turnover, and assure fair bidding for inexpensive to maximize operations.(4) Over the years, attempts to market pharmaceuticals to doctors have gained increasing attention. Pharmaceutical industries use a variety of tactics to increase medicine adoption, such as physician-targeted marketing and other presents.(5) To examine the collision of pharmaceutical marketing strategies on the ethical perceptions of both healthcare professionals and the patients, the key factors, such as DTCA, FI, TDI, RC, PT, TPM, and TPI assessing their influence on trust and ethical concerns while recommending enhancements in regulatory frameworks, was recognized.
The research calculated the ethical difficulties in aesthetic surgery, asking whether it is a medical necessity or a profit-driven enterprise.(6) Findings indicate an identity crisis, as commercialization risks converting the field to a beauty industry that prioritizesfinancial gain over patient well-being. The integrity of the profession was undermined by some surgeons who adhere to ethical standards, while others employ aggressive marketing strategies. The subjective division between medical necessity and elective procedures, as well as different cultural perspectives, was among the limitations. The research emphasized the need to restore core medical values, ensuring that aesthetic surgery remains patient-centered rather than profit-driven. The laws governing the compassionate use of illegal substances in different countrieswas examined.(7) The outcomes draw awareness to important ethical issues, such as fair patient selection and informed consent. Furthermore, ethical frameworks for concerned therapies were provided. Limitations, however, include unpredictable regulations and real-world execution issues in various geographical areas. By exploring prescribing challenges in various countries, the investigation aimed to establish the ethical considerations of Antimicrobial Resistance (AMR).(8) The outcomes demonstrate how national characteristics impact ethical concerns, such as recommending autonomy, group effort, and moral duties to future generations. The results specify that well-organized stewardship applications involve a grasp of country contexts. The complexity of cultural and economic variances, which could affect generalizability, was one of the limitations. Addressing these moral issuesmight support establishing a balance between short-term patient care and long-term public health objectives. The function of the research was to examine the occurrence and effects of DTCA, a topic that has establishedslight attention in scholarly research, on Pakistani consumers' purchasing decisions for over-the-counter (OTC) medications.(9) The findings showed that, regardless of regulatory limitations, DTCA was frequently used to sell unregistered medications, even though it has a key impact on consumer perceptions and needs recognition. In-depth interviews with important parties and meta-synthesis were utilized in the research to shed light on the moral and legal issues surrounding DTCA. The use of qualitative methodologies, which might not accurately reflect the wider customer response, was a significant drawback. The DTCA should be responsibly adopted by pharmaceutical and healthcare professionals for proficient medicine promotion. The investigation examined the primary causes of corruption in pharmaceutical acquisition as well as methods for improving accountability, transparency, and anti-corruption measures.(10) The various levels of government and healthcare systems were prejudiced by the procurement process's high susceptibility to corruption. Risks can be reduced by enhancing accountability and transparency through practices like integrity agreements and open contracting. The research’sdependence on a narrative estimation of the research, which includes both peer-reviewed and grey research, can restrict the thoroughness of the results.
Hypothesis Framework
Hypothesis 1 (H1): DTCA positively influences the EPPM
The technique of marketing prescription products directly to consumers through print, digital, and television media is known as DTCA. It desires to educate individuals about medical procedures along with promoting patient interaction and dialogue with medical professionals.
Hypothesis 2 (H2): FI to healthcare providers negatively influences the EPPM
Pharmaceutical industries give financial incentives to healthcare personnel in terms of bonuses, commissions, or gifts in an attempt to alter prescription procedures. These incentives might lead to a clash of interests; involving financial gain takes precedence over the welfare of patients in medical decisions. By eroding confidence in medical practitioners and the sector, this behavior has a detrimental impact on the ethical perception of pharmaceutical marketing.
Hypothesis 3 (H3): Increased TDI positively influences the EPPM
The open and accurate discovery of a drug's benefits, risks, cost, and clinical trial data is known as improved precision of drug information. This enhances the ethical perception of pharmaceutical marketing by removing misinformation and promoting trust, which in turn assists patients and healthcare providers make informed decisions based on objective information.
Hypothesis 4 (H4): Higher RC by pharmaceutical companies positively influences the EPPM
The pharmaceutical industries can achieve better regulatory compliance by strictly following the rules, regulations, and moral principles that control the creation, promotion, and sale of drugs. Observance to regulations guarantees equitable procedures, precise medication details, and the avoidance of deceptive advertising. This promotes honesty, responsibility, and public confidence, all of which have a beneficial ethical impact on pharmaceutical marketing.
Hypothesis 5 (H5): PT in the pharmaceutical industry positively influences the EPPM
To examine how the ethical perspective of pharmaceutical marketing is improved by public trust in the pharmaceutical sector. Analyzing elements that promote trust, such as openness, patient-centered procedures, and corporate responsibility. Improving public trust can result in more people accepting pharmaceutical marketing as morally just and advantageous.
Hypothesis 6 (H6): The EPPM is positively correlated with the overall TPI
To observe the way broad trust in the pharmaceutical industry is associated with the ethical perception of pharmaceutical marketing. Examining the contact of ethical marketing strategies on the public's trust in pharmaceutical firms is a branch. Gaining insight into this relationship can aid in the development of tactics to improve industry reputation and ethical standards.
These hypotheses form the foundation for the subsequent statistical analysis, as shown in figure 1, which examines the direct, and interaction effects among the independent and dependent variables in the research.
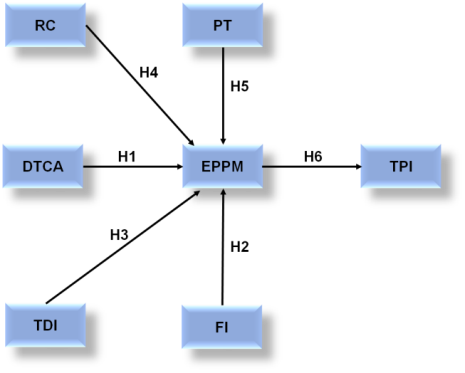
Figure 1. Hypothesis Framework
METHOD
In A cross-sectional survey of 306 Healthcare Professionals and 216 patients was employed in their research to evaluate ethical opinions on pharmaceutical marketing. Participants were chosen using stratified random sampling and convenience. DTCA, financial incentives, openness, regulatory compliance, and public trust were among the important features examined. Structured questionnaires were used to collect data, and SPSS 25 was used for both descriptive statistics and regression analysis. The results provide insight into how patients' and experts' ethical perspectives are influenced by pharmaceutical marketing. Figure 2 illustrates the entire process of the method.
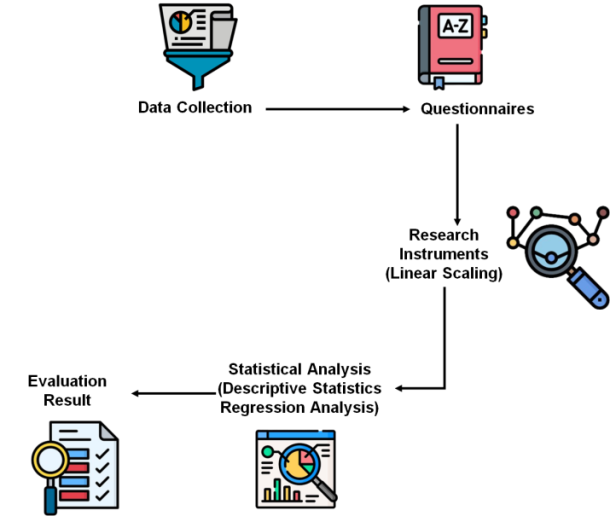
Figure 2. Flow of methodology
Sampling
Patients and medical professionals were the research’s target population. In addition to 216 patients who are undergoing or have received pharmacological therapies, 306 healthcare professionals, including physicians, and pharmacists, are currently in practice. Depending on how easily accessible the target groups are, either convenience sampling or stratified random sampling might be used as the sampling method. Convenience sampling can be used if participant access is restricted; however, stratified random sampling might be utilized to guarantee representation across various patient demographics and professional positions.
Data collection
A cross-sectional survey was conducted to assess the ethical perception of pharmaceutical marketing among healthcare professionals and patients. A sample of 522 people, consistingof 306 medical professionals and 216 patients, participated in the research. DTCA, financial incentives for healthcare providers, drug information transparency, regulatory compliance, and public trust in the pharmaceutical industry were among the marketing strategies used. Measures of respondents' views on ethical concerns, industry trust, and regulatory framework awareness were also incorporated in the poll. The professional role, exposure to pharmaceutical marketing, prior understanding of legislation, direct connection with pharmaceutical personnel, and belief in industry directness were the seven main factors that influenced ethical judgments.
Questionnaires
The examination evaluates clinical experts' and patients' ethical views on pharmaceutical marketing. RC, TDI, PT, FI, EPM, DTCA, and TPI are its seven key constituents. Participants' answers are gathered using open-ended questions. Demographic information serves as a control variable to guarantee thorough analysis. Responses help determine how marketing tactics impact moral perceptions. Table 1 represents the questionnaire based on the key factors and the collected data helps to understand how pharmaceutical marketing impacts public and professional trust.
|
Table 1. Factor-based Questionnaire |
|
|
Factors |
Questionnaires’ |
|
DTCA |
Do you believe DTCA provides accurate drug information? |
|
Do DTCA campaigns influence patient demand for specific medications? |
|
|
FC |
Do financial incentives influence healthcare providers' prescription choices? |
|
Should financial relationships between doctors and pharmaceutical companies be disclosed? |
|
|
TDI |
Do pharmaceutical companies provide clear and honest drug information? |
|
Do you think drug risks are communicated as clearly as drug benefits? |
|
|
RC |
Are pharmaceutical marketing practices sufficiently regulated? |
|
Do you believe existing regulations effectively prevent unethical marketing? |
|
|
PT |
How much do you trust pharmaceutical companies? |
|
Do past controversies in the pharmaceutical industry affect your trust? |
|
|
EPPM |
What is your overall perception of pharmaceutical marketing ethics? |
|
What do you think is the most concerning ethical issue in pharmaceutical marketing? |
|
|
TPI |
Do you believe pharmaceutical companies prioritize public health over profits? |
|
What factors most influence your trust in pharmaceutical companies? |
|
Variables and measurements
The research evaluates significant contributing features to examine how pharmaceutical marketing was apparent ethically. It discovers the effects on ethical judgments of DTCA, financial incentives, transparency, regulatory compliance, and public confidence. Demographics are considered as control factors to ensure an in-depth investigation. Insights into how marketing tactics influence public and professional ethical opinions are offered by the findings.
Independent Variables (IVs)
The following factors are examined for their influence on ethical perception.
DTCA: Examines how marketing targeted directly at consumers (e.g., TV ads for prescription drugs) influences their perception of pharmaceutical ethics.
FI: Investigates whether payments or incentives given to doctors and other healthcare professionals affect their ethical views on pharmaceutical marketing.
TDI: Assesses whether clear, honest communication about drug benefits and risks impacts ethical perceptions.
RC: Evaluates the role of following legal and ethical marketing guidelines in shaping perceptions of ethical conduct.
PT: It looks at how much the public trusts pharmaceutical companies and how that trust affects ethical perceptions.
TPI: Evaluates the extent to which public confidence in pharmaceutical companies influences ethical perceptions of pharmaceutical marketing. More trust levels might lead to a more positive ethical perception, while skepticism or past controversies can contribute to ethical concerns.
Dependent Variable (DV)
EPPM: It reflects how healthcare professionals and patients perceive the ethical nature of pharmaceutical marketing practices.
Data analysis
The IBM SPSS version of 25, a statistical tool frequently employed for research analysis, was used to estimate the data. Demographic information and survey results were gathered using descriptive statistics, which contain means, standard deviations, percentages, and frequencies. The relationship between ethical judgments and important determinants, such as professional function, exposure to pharmaceutical marketing, regulatory knowledge, interactions with representatives, and trust in industry transparency, was considered using regression analysis. To determine how these characteristics affected the ethical attitudes of pharmaceutical marketing, statistical significance was assessed, undertaking accurate and fact-based findings.
RESULTS
The outcomes show a strong connection between pharmaceutical marketing tactics and patients' and healthcare professionalsethical perspectives. While financial incentives provide away to ethical issues, transparency and regulatory compliance have a beneficial impact on ethical attitudes. These impacts are restrained by public trust, underscoring its critical role in the construction of perceptions. These results support the goal of the research, which is to comprehend how ethical opinions are influenced by marketing strategies.
Demographic Table
The analysis of the demographic data presents an approach into the characteristics of the participants regarding ethical perceptions in pharmaceutical marketing. The sample contains an elevated proportion of females than males along with both healthcare professionals and patients. The age distribution is well-balanced, with a significant portion of participants in the middle-age category, while younger and older groups are also fairly represented. Educational backgrounds vary, but the majority holds a bachelor’s degree, followed by associates, masters, and doctoral degrees. In terms of experience, while participants are distributed across different professional experience levels, a notable portion has 0-5 years of experience, indicating a fair representation of early-career professionals. Table 2 illustrates the demographic characteristics of the participants and figure 3 shows the graphical representation of the demographic variables.
|
Table 2. Demographic characteristics of the participants |
|||||
|
Demographic Variable |
Category |
Healthcare Professionals (n=306) |
Percentage (%) |
Patients (n=216) |
Percentage (%) |
|
Gender |
Female |
146 |
47,71 |
96 |
44,44 |
|
Male |
160 |
52,29 |
120 |
55,56 |
|
|
Age Group (years) |
18-25 |
52 |
16,99 |
56 |
25,93 |
|
26-35 |
110 |
35,95 |
42 |
19,44 |
|
|
36-45 |
86 |
28,10 |
79 |
36,57 |
|
|
46+ |
58 |
18,95 |
39 |
18,06 |
|
|
Educational Level |
Associate Degree |
105 |
34,31 |
68 |
31,48 |
|
Bachelor’s Degree |
95 |
31,05 |
52 |
24,07 |
|
|
Master’s Degree |
55 |
17,97 |
46 |
21,30 |
|
|
Doctoral Degree |
51 |
16,67 |
50 |
23,15 |
|
|
Experience Level |
0-5 years |
109 |
35,62 |
79 |
36,57 |
|
6-10 years |
72 |
23,53 |
58 |
26,85 |
|
|
11-20 years |
68 |
22,22 |
40 |
18,52 |
|
|
21+ years |
57 |
18,63 |
39 |
18,06 |
|
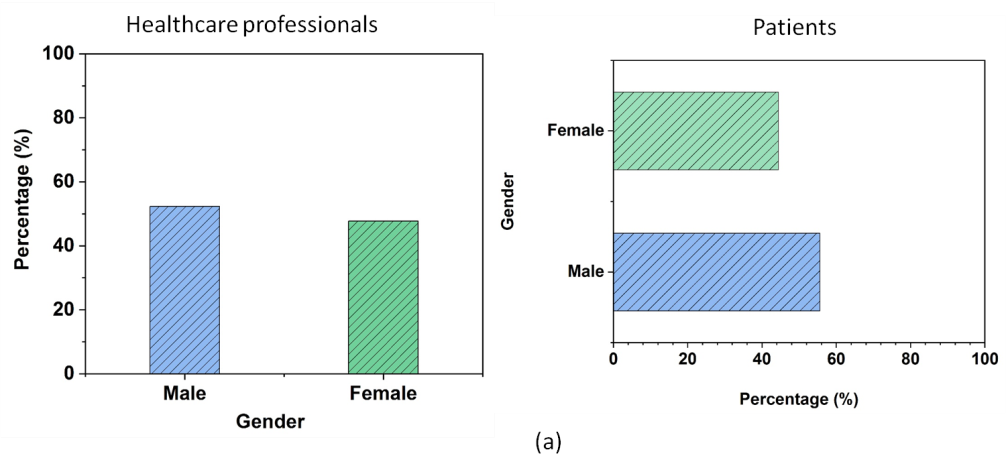
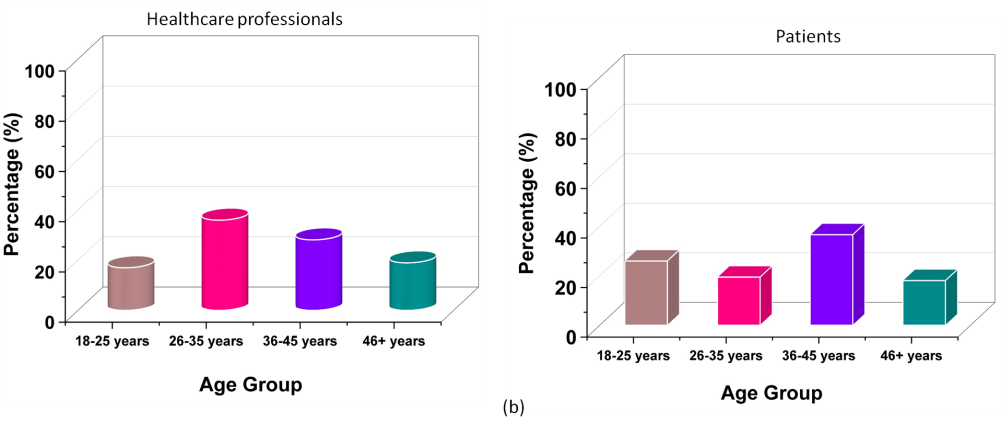
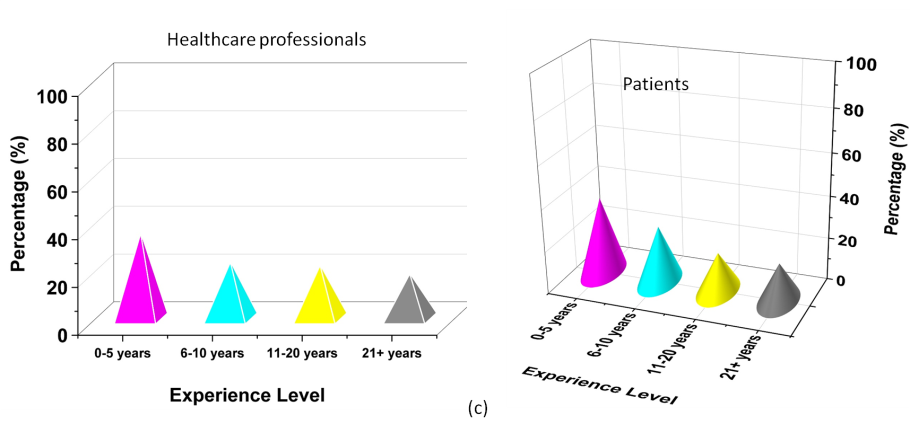
Figure 3. A graphical representation of demographic factors Gender (a), Age Group (b), Experience Level (c)
Descriptive statistics
In pharmaceutical marketing, descriptive statistics are employed to assess important facets of ethical judgments. Participants' average perception score is modest, and the significant standard deviation (SD) suggests that answers varied. According to statistical testing, ethical opinions are strongly influenced by transparency and regulatory compliance, although the impacts of financial incentives are not entirely clear. Furthermore, a high degree of favorability toward open communication points to its influence on moral opinions. Overall, the results demonstrate how crucial marketing tactics are in shaping ethical attitudes.
![]()
Equation (1) aligns with the objective by computing variations in ethical insights of pharmaceutical industry. Here, w represents the data value, w ̅ is the average of the collected data, m-1 denotes the adjusted sample size, and |w-w ̅ |2 captures deviations from the mean. This statistical approach helps assess the impact of transparency, financial incentives, and regulatory compliance on ethical perceptions, supporting the research goal of understanding how marketing practices shape public and professional views. The descriptive statistics of key variables were listed in table 3 and the graphical illustrations were demonstrated in figure 4.
|
Table 3. Descriptive statistics of Pharmaceutical Marketing Factors and Public Trust |
||||||
|
Variables |
Group |
Mean |
SD |
Min |
Max |
Median |
|
DTCA |
Healthcare Professionals |
3,8 |
0,9 |
1,5 |
5,0 |
3,9 |
|
Patients |
4,2 |
1,0 |
1,8 |
5,0 |
4,3 |
|
|
EEPM |
Healthcare Professionals |
4,0 |
0,8 |
2,0 |
5,0 |
4,1 |
|
Patients |
3,7 |
1,1 |
1,3 |
5,0 |
3,7 |
|
|
FI |
Healthcare Professionals |
3,5 |
1,1 |
1,0 |
5,0 |
3,6 |
|
Patients |
3,9 |
1,2 |
1,2 |
5,0 |
4,0 |
|
|
TDI |
Healthcare Professionals |
4,1 |
0,8 |
2,0 |
5,0 |
4,2 |
|
Patients |
3,7 |
1,0 |
1,5 |
5,0 |
3,8 |
|
|
RC |
Healthcare Professionals |
4,3 |
0,7 |
2,5 |
5,0 |
4,4 |
|
Patients |
3,8 |
1,1 |
1,5 |
5,0 |
3,9 |
|
|
PT |
Healthcare Professionals |
3,7 |
1,0 |
1,5 |
5,0 |
3,8 |
|
Patients |
4,0 |
1,1 |
1,2 |
5,0 |
4,1 |
|
|
TPI |
Healthcare Professionals |
4,0 |
0,9 |
2,0 |
5,0 |
4,1 |
|
Patients |
3,6 |
1,2 |
1,3 |
5,0 |
3,7 |
|
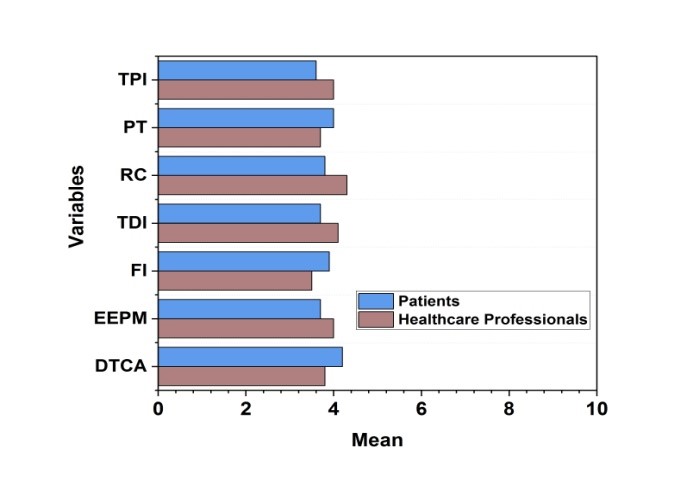
Figure 4. A graphical representation of Descriptive statistics
Regression analysis
A statistical method used to examine relationships between independent and dependent variables was regression analysis. It helps in understanding how factors such as transparency, regulatory compliance, and ethical perception influence contract enforceability. The analysis identifies key predictors, measures their significance, and provides empirical evidence for policy formulation. The regression equation is expressed inequation (2).
![]()
Where b is the intercept, X is the dependent variable, y represents the independent variable, a represents the beta coefficient, and v depicts the error term. Table 4 depicts the key variables in regression analysis.
|
Table 4. Regression Analysis of Factor Influencing Ethical Perception of Pharmaceutical Marketing |
|||||
|
Factors |
Coefficient |
p-value |
95 % CI (Lower, Upper) |
t-value |
Std. Error |
|
DTCA |
0,22 |
0,000 |
(0,12, 0,32) |
4,40 |
0,05 |
|
EPPM |
-0,15 |
0,033 |
(-0,29, -0,01) |
-2,14 |
0,07 |
|
FI |
0,35 |
0,000 |
(0,23, 0,47) |
5,83 |
0,06 |
|
TDI |
0,27 |
0,000 |
(0,19, 0,35) |
6,75 |
0,04 |
|
RC |
0,25 |
0,000 |
(0,15, 0,35) |
5,00 |
0,05 |
|
PT |
0,30 |
0,000 |
(0,20, 0,40) |
6,00 |
0,05 |
|
TPI |
0,22 |
0,000 |
(0,12, 0,32) |
4,40 |
0,05 |
These outcomes demonstrate how economic and moral considerations have an effect on the compliance of contracts, which helps rigid and regulators make wise choices.
Path analysis
A statistical method for examining both direct and indirect links between research variables is path analysis. The seven variables and their effects on public trust in the pharmaceutical sector are estimated using path analysis. The outcomes show that while financial incentives have a harmful effect on ethical perception, DTCA, transparency, and regulatory compliance have anoptimistic effect. Additionally, ethical perception is deeply influenced by public trust, which in turn enhances overall trust in the pharmaceutical sector. The research offers perceptive information on improving industry credibility and ethical marketing strategies. Table 5 represents the results of pathway analysis.
|
Table 5. Pathway Analysis |
||||||
|
Hypothesis |
Pathway |
β |
t-value |
SE |
p-value |
Accepted / Not Accepted |
|
H1 |
DTCA → EPPM |
0,32 |
4,57 |
0,07 |
<0,001 |
Accepted |
|
H2 |
FI → EPPM |
-0,28 |
-4,67 |
0,06 |
<0,001 |
Accepted |
|
H3 |
TDI → EPPM |
0,41 |
8,20 |
0,05 |
<0,001 |
Accepted |
|
H4 |
RC → EPPM |
0,35 |
5,83 |
0,06 |
<0,001 |
Accepted |
|
H5 |
PT → EPPM |
0,38 |
7,60 |
0,05 |
<0,001 |
Accepted |
|
H6 |
EPPM → TPI |
0,45 |
11,25 |
0,04 |
<0,001 |
Accepted |
The test findings for various routes and their effects on EPPM and TPI are shown in the table. The findings indicate that, depending on the pathway's orientation, DTCA, FI, TDI, RC, and PT all have a substantial influence on EPPM, with either positive or negative coefficients. Furthermore, EPPM has a significant effect on TPI, which emphasizes the importance of emotional processing in influencing public perception and trust. Since every p-value was below the 0,001 threshold, confirming the strength of the correlations between the variables, all of the hypotheses were accepted.
CONCLUSIONS
The research provides a general investigation of the ethical considerations in pharmaceutical marketing, representing the way ethical attitudes are impacted by the seven variables. The results indicate that while increased sincerity and regulatory compliance enhance industry trust, financial incentives might be a causal factor to ethical issues. Regression analysis highlights the constraint for more severe laws and moral marketing practices by confirming the well-built connection between marketing methods and ethical perception. Enhancing compliance and openness can increase public trust and assurance a more ethical pharmaceutical sector. To further improve industry credibility, future research should observe regional differences and the long-term impacts of ethical marketing reforms.
BIBLIOGRAPHIC REFERENCES
1. Ahmed RR, Channar ZA, Soomro RH, Vveinhardt J, Streimikiene D, Parmar V. Antecedents of symmetry in physicians’ prescription behavior: evidence from SEM-based multivariate approach. Symmetry. 2018 Dec 5;10(12):721. https://doi.org/10.3390/sym10120721
2. Prasad M, Lambe UP, Brar B, Shah I, Manimegalai J, Ranjan K, Rao R, Kumar S, Mahant S, Khurana SK, Iqbal HM. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomedicine & Pharmacotherapy. 2018 Jan 1; 97:1521-37. https://doi.org/10.1016/j.biopha.2017.11.026
3. Siyal AA, Junejo AZ, Zawish M, Ahmed K, Khalil A, Soursou G. Applications of blockchain technology in medicine and healthcare: Challenges and future perspectives. Cryptography. 2019 Jan 2;3(1):3. https://doi.org/10.3390/cryptography3010003
4. Lee, D. and Yoon, S.N., 2021. Application of artificial intelligence-based technologies in the healthcare industry: Opportunities and challenges. International journal of environmental research and public health, 18(1), p.271. https://doi.org/10.3390/ijerph18010271
5. Khazzaka M. Pharmaceutical marketing strategies’ influence on physicians’ prescribing pattern in Lebanon: ethics, gifts, and samples. BMC health services research. 2019 Dec;19:1-1. https://doi.org/10.1186/s12913-019-3887-6
6. Atiyeh BS, Rubeiz MT, Hayek SN. Aesthetic/cosmetic surgery and ethical challenges. Aesthetic plastic surgery. 2020 Aug;44:1364-74. https://doi.org/10.1007/s00266-020-01821-z
7. Borysowski J, Górski A. Compassionate use of unauthorized drugs: legal regulations and ethical challenges. European Journal of Internal Medicine. 2019 Jul 1;65:12-6. https://doi.org/10.1016/j.ejim.2019.04.008
8. Krockow EM, Tarrant C. The international dimensions of antimicrobial resistance: Contextual factors shape distinct ethical challenges in South Africa, Sri Lanka and the United Kingdom. Bioethics. 2019 Sep;33(7):756-65. https://doi.org/10.1111/bioe.12604
9. Qureshi JA, SZABIST K, Qureshi MA, Shah Z, Ahmed A, MNS-UET MA. Direct-to-Consumer Advertising (DTCA) in Pharmaceutical Industry: An Inquiry from Public Policy to Practice and Consumers’ Perspective. http://dx.doi.org/10.1509/jppm.21.2.213.17592
10. Kohler JC, Dimancesco D. The risk of corruption in public pharmaceutical procurement: how anti-corruption, transparency and accountability measures may reduce this risk. Global health action. 2020 Feb 3;13 (sup1):1694745. https://doi.org/10.1080/16549716.2019.1694745
FINANCING
No financing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Data curation: Malathi H, Rohini, Laxmidhar Maharana.
Methodology: Malathi H, Rohini, Laxmidhar Maharana.
Software: Malathi H, Rohini, Laxmidhar Maharana.
Drafting - original draft: Malathi H, Rohini, Laxmidhar Maharana.
Writing - proofreading and editing: Malathi H, Rohini, Laxmidhar Maharana.