doi: 10.56294/mw2023129
ORIGINAL
Health Informatics Systems for Information and Decisional Control in Cancer Patients’ Medical Decision-Making
Sistemas informáticos sanitarios para el control informativo y decisorio en la toma de decisiones médicas de pacientes con cáncer
Soumya Surath Panda1 ![]() *, Manashree
Mane2
*, Manashree
Mane2 ![]() , Sourabh Kumar Singh3
, Sourabh Kumar Singh3 ![]()
1IMS and SUM Hospital, Siksha ‘O’ Anusandhan (deemed to be University), Department of Onco-Medicine. Bhubaneswar, Odisha, India.
2JAIN (Deemed-to-be University), Department of Forensic science. Bangalore, Karnataka, India.
3Department of Forensic Science, Noida International University. Greater Noida, Uttar Pradesh, India.
Cite as: Panda SS, Mane M, Singh SK. Health Informatics Systems for Information and Decisional Control in Cancer Patients’ Medical Decision-Making. Seminars in Medical Writing and Education. 2023; 2:129. https://doi.org/10.56294/mw2023129
Submitted: 12-09-2022 Revised: 24-12-2022 Accepted: 27-02-2023 Published: 28-02-2023
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding Author: Soumya Surath Panda *
ABSTRACT
Health Informatics Systems (HIS) are technology-based solutions that enhance healthcare efficiency by collecting, managing, and analyzing health data, including electronic health records, clinical decision support systems, and telemedicine platforms. HISs utilize technology to manage healthcare data, improve patient care, and enhance decision-making. The aim is to develop and evaluate HIS that enhances information management and decisional control in cancer patients’ medical decision-making, ensuring informed and patient-centered care. The research surveyed 412 individuals who had been diagnosed with cancer. To ensure a diverse patient population, participants were selected from multiple healthcare institutions. The analysis utilized the Control Preferences Scale (CPS) to assess patients’ preferred level of involvement in medical decisions. Patients were grouped into four decision-making categories: self-reliant, guided decision-making, co-decision, and non-participatory. The data was analyzed using SPSS 26 software to guarantee methodological rigor and reliability. Descriptive statistics, logistic regression, and analysis of variance (ANOVA) compare means across multiple groups to determine whether significant differences exist in decision-making preferences among cancer types. Self-reliance and guided decision-making are prevalent in the early stages, while non-participatory decision-making increases in the advanced stages. Logistic regression shows significant associations between HIS usage and decision-making styles. ANOVA confirms statistical differences in decision-making approaches across different patient groups. The findings highlight the diverse decision-making preferences among cancer patients, emphasizing the need for tailored HIS that support informed, and patient-centered care. Enhancing real-time data access and predictive insights can empower patients and improve collaborative decision-making in oncology.
Keywords: Health Informatics Systems (HIS); Cancer Patients; Decision-Making; Medical Advice; Decisional Control.
RESUMEN
Los sistemas informáticos de salud (HIS, por sus siglas en inglés) son soluciones tecnológicas que mejoran la eficiencia de la atención sanitaria mediante la recopilación, la gestión y el análisis de datos sanitarios, como las historias clínicas electrónicas, los sistemas de apoyo a la toma de decisiones clínicas y las plataformas de telemedicina. Los HIS utilizan la tecnología para gestionar los datos sanitarios, mejorar la atención al paciente y potenciar la toma de decisiones. El objetivo es desarrollar y evaluar HIS que mejoren la gestión de la información y el control decisorio en la toma de decisiones médicas de los pacientes con cáncer, garantizando una atención informada y centrada en el paciente. La investigación encuestó a 412 personas a las que se había diagnosticado cáncer. Para garantizar una población de pacientes diversa, los participantes fueron seleccionados de múltiples instituciones sanitarias. El análisis utilizó la Escala de Preferencias de Control (EPC) para evaluar el nivel preferido de participación de los pacientes en las decisiones médicas. Los pacientes se agruparon en cuatro categorías de toma de decisiones: autosuficiente, toma de decisiones guiada, codecisión y no participativa. Los datos se analizaron con el programa SPSS 26 para garantizar el rigor metodológico y la fiabilidad. Las estadísticas descriptivas, la regresión logística y el análisis de la varianza (ANOVA) comparan las medias de varios grupos para determinar si existen diferencias significativas en las preferencias de toma de decisiones entre los distintos tipos de cáncer. La autosuficiencia y la toma de decisiones guiada prevalecen en los estadios iniciales, mientras que la toma de decisiones no participativa aumenta en los estadios avanzados. La regresión logística muestra asociaciones significativas entre el uso de HIS y los estilos de toma de decisiones. El ANOVA confirma las diferencias estadísticas en los enfoques de toma de decisiones entre los distintos grupos de pacientes. Los resultados ponen de manifiesto la diversidad de preferencias en la toma de decisiones de los pacientes con cáncer, lo que subraya la necesidad de sistemas de información sanitaria personalizados que apoyen una atención informada y centrada en el paciente. La mejora del acceso a los datos en tiempo real y de la información predictiva puede empoderar a los pacientes y mejorar la colaboración en la toma de decisiones en oncología.
Palabras clave: Sistemas Informáticos de Salud (HIS); Pacientes con Cáncer; Toma de Decisiones; Asesoramiento Médico; Control Decisional.
INTRODUCTION
Technologies to gather, organize, and evaluate health information, medical information structures, or HIS, are essential to modern medical services. By increasing information accessibility and decision-making effectiveness, these systems, which include clinical decision support systems (CDSS), electronic health records (EHR), and telemedicine platforms, improve healthcare for patients.(1) By assisting clients and medical professionals with generating evidence-based decisions, HIS can have a substantial impact on medical outcomes in oncology, where prompt and informed decisions are essential. But even with the progress in health informatics, many cancer patients still have trouble making decisions. They frequently have difficulty understanding their treatment options, evaluating the risks, and deciding how much influence they want to have over their care.(2) The methods used currently to help cancer patients make treatment decisions differ greatly. In previous generations, doctors made the majority of the decisions about therapy, and patients followed their suggestions. The practice of patient-centered medicine has evolved over the years doctor’s work together with patients to select treatment strategies.(3) Some obstacles remain despite every individual healthcare patient pursuing active involvement. Active involvement in healthcare choices exists among some people but others choose to defer medical decisions to doctors due to medical complexities.(4) However, challenges remain, each patient wants to be as involved to different extent. While some prefer to actively participate in their healthcare decisions, others may feel overburdened by the complexities of medicine and would rather rely on their doctors for advice.(5) Figure 1 shows the patients' medical decision-making.
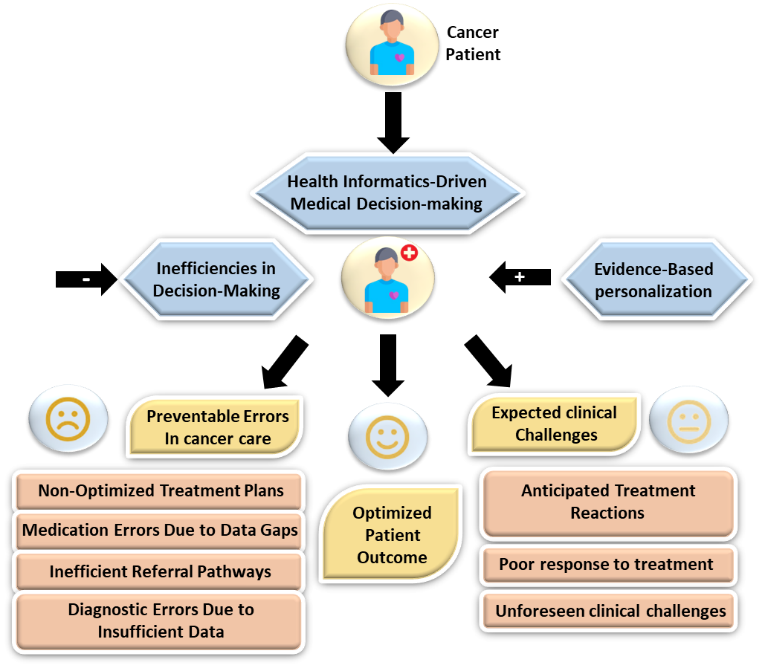
Figure 1. Overview of Patients' Medical Decision-Making
The current solutions fail to unite with advanced analytics solutions that would generate predictions to increase patient engagement. To build and assess HIS capable of enhancing both cancer patient information managing and decision- making capabilities.(6) Real-time data access, predictive analytics, and personalized decision-support enable the proposed HIS to give patients targeted control levels throughout different cancer types and demographic populations.(7) The investigation shows that patients require suitable motivation to stress the importance of developing innovative HISs that enhance decision-making skills leading to improved cancer treatment outcomes.(8) This examination used a planned authorized experiment to evaluate the effect of the decision-making approach (DA) on CRC screening outcomes at primary healthcare facilities.
The different variables, including decisional conflict together with disease knowledge as well as test-taking intention and goal-intention concordance were examined.(9) The decision aid proved more suitable in clinic A because knowledge levels and levels of decision conflict showed substantial differences (p<0,001). In clinic B, only significant variations were in knowledge (p<0,001). During digital Personal Patient profile–prostate (P3P) education and decision-making planning, a randomized trial compared the standard healthcare and intervention teams. To examine relationships between variables, such as age, education, marital status, insurance, and decision-making stage, participants were interviewed using Tobit models and assigned either high or moderate initial levels were evaluated.(10) The final multivariable Tobit regression model showed a significant relationship between willingness to pay (WTP) for P3P and low Starting Value (SV), income, marital status, and decision-making stages. A preference for decision-making freedom was considered moderately significant (p=0,11).
The investigation examined the relationships between Health-Related quality of life (HRQOL), major depressive disorder risk, and treatment decisions in patients aged 65 and older, who were diagnosed with first-stage non-small cell lung cancer (NSCLC) between 2004 and 2013.(11) These patients participated in the health effects assessment within 24 months before diagnosis. A lower chance to receive radiotherapy (RT) as opposed to surgical treatment was linked to improved overview of the Physical Component summary(PCS) and Mental Component Summary (MCS) outcomes on multivariable analyses (modified probability coefficient per ten-element PCS enhance, 0,60 [95 % cl, 0,45 0,79; P, 0,001]; modified possibility coefficient per ten-elements MCS enhance, 0,61 [95 % CI 0,46 to 0,80; P,0,001]). A trial evaluating P3P decision-making interventions focused on assigning men with low-risk cancer to P3P instead of standard care and providing them with recommended treatments. For these males, the probability of the desired outcome was calculated using a multivariable system presented.(12) After employing P3P, more males in low-risk patients sought active monitoring, regardless of whether participants were offered limited alternatives (71 % vs. 59 % with usual care; P =0,34) or unlimited possibilities (62 % vs. 54 % with usual care; P =0,54).
For a planned prostate biopsy, 1028 males from 4 VA hospitals were enlisted.(13) After completing benchmark assessments, participants remained shuffled to obtain a basic and complicated decision aid. Contributors included male patients who had completed a baseline survey and had medically specific cancer (N=285) by biopsy. After reading the DA, participants who received the basic DA were more interested in shared decision-making (p=0,03), found the DA more useful (p<0,01), and were more likely to consider monitoring (p=0,03) than those who received the multifaceted decision aid at time two.
Depression, anxiety, quality of life, global health, post-traumatic coping, and material adjustment in 52 advanced cancer patients (ACP-SC) couples were surveyed prospectively about their feelings and expectations regarding therapy investigated.(14) Recurrence (p = 0,04), shrinkage (p = 0,01), and stabilization (p = 0,01) were observed. Additionally, regression analysis shows a negative correlation between SC anxiety states (p = 0,01) and traits (p = 0,02) and spousal caregivers' (SC) expectations for stability. The principal finding of a randomized controlled trial (RCT) comparing genomics advisers and genetic counseling for secondary findings (SF) selection was a decision struggle. Participants were randomly assigned to use either the decision aid (DA), genomic counseling, or both. In contrast, involved participants had significantly shorter guidance times (24,40 minutes less; P < 0,001) and demonstrated a greater understanding of SF (0,039; P< 0,001) and sequencing benefits (0,097; p = 0,01).(15) To ensure informative and patient-centered treatment, the goal is to create and assess HIS that improves information management and decision control in cancer patients’ medical decision-making.
METHOD
Investigation uses a cross-sectional questionnaire to assess cancer patients' preferences for using Health Informatics Systems (HIS) to make decisions. Patients are categorized by their engagement in healthcare decisions using the Control Preferences Scale (CPS). SPSS 26 technology is used to perform statistical analysis, such as chi-square tests, logistic regression, and ANOVA, to find trends and evaluate how well HIS supports patient-centered decision-making.
Data collection
The study collected data from 412 cancer patients across multiple healthcare institutions to ensure a diverse patient population. Participants were selected using stratified random sampling, considering cancer type, age, and demographics. A structured survey questionnaire incorporating the Control Preferences Scale (CPS) was used to assess patients’ decision-making preferences. Data were gathered through in-person and online surveys, with responses categorized into four groups: self-reliant, Guided Decision-Making, guided, Co-Decision Making, and non-participatory (10 %). Research collected additional demographics, cancer type, gender, age group, and decision-making preference to examine trends and correlations. Figure 2 and table 1 show the demographic information.
|
Table1. Demographic data |
|||
|
Variable |
Category |
Frequency (n = 412) |
Percentage (%) |
|
Gender |
Male |
180 |
43,70 |
|
Female |
232 |
56,30 |
|
|
Age Group |
<30 |
50 |
12,10 |
|
30-50 |
190 |
46,10 |
|
|
>50 |
172 |
41,80 |
|
|
Education Level |
No Formal Education |
40 |
9,70 |
|
High School |
170 |
41,30 |
|
|
College/University |
202 |
49,00 |
|
|
Cancer Type |
Breast Cancer |
120 |
29,10 |
|
Lung Cancer |
90 |
21,80 |
|
|
Colorectal Cancer |
70 |
17,00 |
|
|
Others |
132 |
32,10 |
|
|
Decision-Making Preference |
Self-Reliant |
110 |
26,70 |
|
Guided Decision-Making |
140 |
34,00 |
|
|
Co-Decision |
90 |
21,80 |
|
|
Non-Participatory |
72 |
17,50 |
|

Figure 2. Overview of Demographic
Structure questionnaires
Self-Reliant: in this section, patients want to be employed evenly among physicians or make healthcare treatments on their own. It takes charge of their medical decisions, actively seeks out information, and weighs possibilities for treatments.
Guided Decision-Making: After consulting with medical professionals, the patient makes decisions based on expert recommendations, while still maintaining flexibility in their final choices despite valuing medical advice.
Co-Decision: clients work with physicians to prepare their treatments and prefer shared decision-making. It is to confer, weigh their own opinions against medical knowledge, and determine the best course of action together.
Non-Participatory: for medical decisions, these patients entirely depend on their doctors, putting their faith in their professional knowledge. Their preference for minimum involvement is frequently a result of emotional discomfort, a lack of professional experience, or faith in the judgment of medical professionals. The sample questionnaires are shown in table 2.
|
Table 2. Sample Questionnaires |
||
|
Variable |
Number Questions |
Survey Question |
|
Self-Reliant Decision-Making |
3 |
Do you prefer to make your own medical decisions without external influence? |
|
|
How often do you research treatment options before consulting a doctor? |
|
|
|
Do you feel confident in making final decisions about your cancer treatment? |
|
|
Guided Decision-Making |
3 |
Do you prefer receiving medical advice before making a decision? |
|
|
How important is a doctor's recommendation in your final treatment choice? |
|
|
|
Do you rely on healthcare professionals to guide but not decide your treatment? |
|
|
Co-Decision Making |
3 |
Do you prefer to share decision-making equally with your doctor? |
|
|
How often do you discuss multiple treatment options with your doctor before deciding? |
|
|
|
Do you feel that shared decision-making leads to better treatment outcomes? |
|
|
Non-Participatory Decision-Making |
3 |
Do you prefer doctors to make all treatment decisions on your behalf? |
|
|
How comfortable are you with leaving all medical decisions to your doctor? |
|
|
|
Do you feel more secure when doctors take full responsibility for your treatment plan? |
|
Statistical Assessment
Decision-making patterns are examined in this investigation using SPSS 26 software, improving HIS for better information management and patient-centered cancer care. Patient information and interests for decision-making were compiled using descriptive statistics. Analysis of variance (ANOVA) was utilized to compare means among various categories of patients, and logistic regression was employed to determine the factors impacting decision-making styles. A thorough grasp of HIS might improve individualized decision-making in cancer care, made possible by these statistical techniques.
RESULTS
By examining decision-making desires among four variables self-reliant, guided decision-making, co-decision-making, and non-participatory the investigation assesses how HIS can improve information management and decisional control in cancer patients. Utilizing the components in this section, assess the descriptive statistics, logistic regression, and analysis of variance (ANOVA).
Descriptive Statistics
Descriptive Statistics analyze patient demographics, decision-making preferences, and HIS usage, revealing patterns and variations in cancer patient's choices. The main features of the database, such as patient demographics, preferred methods of decision-making, and HIS usage, are summarised using descriptive statistics. Metrix identical mean, median, standard deviation and frequency ranges help identify structures and variations in cancer patients' make medical – decisions equation (1).
![]()
The mean is represented by W, the individual values by Wj, and the total number of values by M. Descriptive statistics for decision-making styles in cancer patients with early and late stages are shown in table 3. Non-participatory decision-making has the lowest mean (2,95, SD = 0,90) in the early stage, while self-reliant decision-making has the highest mean (3,85, SD = 0,75), with a range of 3,10 to 4,60. Non-participatory remaining at its lowest level (mean = 3,20, SD = 0,95), while directed decision-making researches at its peak (mean = 4,10, SD = 0,70) in the advanced stages. The variability in decision-making preferences is indicated by standard deviations. Table 3 represents the outcome of descriptive statistics.
|
Table 3. Result of Descriptive Statistics |
|||||
|
Cancer Stage |
Variable |
Mean |
Standard Deviation |
Maximum |
Minimum |
|
Early Stage |
Self-Reliant Decision-Making |
3,85 |
0,75 |
4,60 |
3,10 |
|
Guided Decision-Making |
3,60 |
0,80 |
4,40 |
2,80 |
|
|
Co-Decision Making |
3,40 |
0,65 |
4,05 |
2,75 |
|
|
Non-Participatory Decision-Making |
2,95 |
0,90 |
3,85 |
2,05 |
|
|
Advanced Stage |
Self-Reliant Decision-Making |
3,70 |
0,85 |
4,55 |
2,85 |
|
Guided Decision-Making |
4,10 |
0,70 |
4,80 |
3,40 |
|
|
Co-Decision Making |
3,75 |
0,80 |
4,55 |
2,95 |
|
|
Non-Participatory Decision-Making |
3,20 |
0,95 |
4,15 |
2,25 |
|
Logistic Regression
To identify key predictors of decisional management, logistic regression analyzes demographics and HIS usage influence the decision-making methods of cancer patients. Logistic regression evaluates the association between variables (such as demographics and HIS usage). It helps identify the variables that influence a patient's likelihood of making healthcare decisions in a participatory, self-reliant, guided, or non-participatory manner.
![]()
O(Z=1) the possibility of this output will materialize (e.g., the patient selecting a particular decision-making style). f Euler's number, or around 2,718. β0 intercept (term constant).
β1, β2,…,βm Coefficients for predictor variables.W1,W2,…, Wm using odds ratios (OR), confidence intervals (CI), and p-values, the table illustrates the relationship between cancer stage and decision-making methods. Early-stage, non-participatory decision-making is the least prevalent (OR: 0,95, p=0,780), but guided decision-making (OR: 1,45, p=0,010) and self-reliant decision-making (OR: 1,85, p=0,002) are more probable. Non-participatory decision-making (OR: 2,10, p=0,001) is more common in the advanced stage, although co-decision-making (OR: 1,50, p=0,015) and self-reliance (OR: 1,40, p=0,030) also rise. Stronger connections are indicated by significant p-values (<0,05). Table 4 and figure 3 represent the outcome of logistic regression.
|
Table 4. Result of Logistic Regression |
||||
|
Cancer Stage |
Variables |
Odds Ratio (OR) |
95 % Confidence Interval (CI) |
p-Value |
|
Early Stage |
Self-Reliant Decision-Making |
1,85 |
1,30 – 2,60 |
0,002 |
|
Guided Decision-Making |
1,45 |
1,10 – 1,90 |
0,010 |
|
|
Co-Decision Making |
1,20 |
0,85 – 1,70 |
0,230 |
|
|
Non-Participatory Decision-Making |
0,95 |
0,60 – 1,50 |
0,780 |
|
|
Advanced Stage |
Self-Reliant Decision-Making |
1,40 |
1,05 – 1,90 |
0,030 |
|
Guided Decision-Making |
1,75 |
1,25 – 2,45 |
0,005 |
|
|
Co-Decision Making |
1,50 |
1,10 – 2,00 |
0,015 |
|
|
Non-Participatory Decision-Making |
2,10 |
1,50 – 3,00 |
0,001 |
|

Figure 3. Result of Logistic regression (a) Early Stage and (b) Advanced Stage
Analysis of variance (ANOVA)
ANOVA assesses differences in cancer patients' decision-making based on demographics, HIS usage, and decisional control preferences. Decision-making preferences across various cancer categories are one example of the way ANOVA evaluates mean differences between numerous groups. Determining if differences in decision-making styles are statistically significant ensures a better understanding of when HIS might be customized to meet the needs of various patient populations, as shown in equation (3).
![]()
NTbetween =mean square between groups (variance due to group differences)
NTwithin =mean square within groups (variance due to individual differences within each group). The analysis of variance (ANOVA) outcomes for various decision-making approaches among cancer stages is displayed in table 5. While non-participatory decision-making (p=0,110) is not significant, self-reliant (MS: 4,25, F: 3,85, p=0,012), guided decision-making (MS: 3,80, F: 4,10, p=0,009), and co-decision-making (MS: 3,50, F: 2,75, p=0,045) all exhibit meaningful variations in the early stage. All decision-making styles exhibit notable variations in the higher stages, with the largest F-value occurring in non-participatory decision-making (MS: 3,20, F:5,10, p=0,003). Table 5 and figure 4 represent the outcome of the Analysis of variance (ANOVA).
|
Table 5. Result of Analysis of variance (ANOVA) |
||||
|
Cancer Stage |
Variable |
Mean Square (MS) |
F-Value |
p-Value |
|
Early Stage |
Self-Reliant Decision-Making |
4,25 |
3,85 |
0,012 |
|
Guided Decision-Making |
3,80 |
4,10 |
0,009 |
|
|
Co-Decision Making |
3,50 |
2,75 |
0,045 |
|
|
Non-Participatory Decision-Making |
2,95 |
1,90 |
0,110 |
|
|
Advanced Stage |
Self-Reliant Decision-Making |
3,90 |
3,20 |
0,018 |
|
Guided Decision-Making |
4,15 |
4,50 |
0,006 |
|
|
Co-Decision Making |
3,70 |
3,85 |
0,011 |
|
|
Non-Participatory Decision-Making |
3,20 |
5,10 |
0,003 |
|

Figure 4. Result of ANOVA (a) Early Stage (b) Advanced Stage
DISCUSSION
By increasing material availability, patient involvement, and collaborative decision-making, HIS improves information management and decisional control in cancer care. Throughout the various phases of cancer and the course of therapy, customized HIS solutions can empower patients, assist physicians, and guarantee informed, patient-centered medical decisions. Higher decision-making outcomes are indicated by the two highest mean values in the descriptive statistics table self-reliant decision-making in early stage (Mean = 3,85, SD = 0,75, Max = 4,60, Min = 3,10) and guided decision-making in advanced stage (Mean = 4,10, SD = 0,70, Max = 4,80, Min = 3,40). The logistic regression table shows the greatest important impacts on decision-making outcomes across cancer stages, with the two strongest associations being non-participatory decision-making in advanced stage (OR = 2,10, 95 % CI: 1,50–3,00, p=0,001) and self-reliant decision-making in early stage (OR = 1,85, 95 % CI: 1,30–2,60, p = 0,002). The two most statistically important differences in decision-making variations across cancer stages are non-participatory decision-making in the advanced stage (F = 5,10, p = 0,003) and guided decision-making in the advanced stage (F = 4,50, p = 0,006), which are the two greatest outcomes of the ANOVA.
CONCLUSIONS
The investigation emphasizes the need for customized Health Informatics Systems (HIS) to improve updated, patient-oriented treatment by highlighting the variety of decision-making preferences among cancer patients. Patients can be empowered by real-time data access and predictive insights, enhance team decision – making and individualized cancer treatment strategies. The probabilities of advanced-stage cancer are considerably increased by Guided Decision-Making (mean 4,10) which suggests decreased dangers of patient engagement. Non-participatory decision-making in the Advanced Stage (F = 5,10, p = 0,003) is the greatest significant test value in this analysis, based on the ANOVA findings. The largest probability for advanced-stage cancer is found in non-participatory decision-making (OR: 2,10,95 CI: 1,50-3,00, p=0,001), underscoring the effect of low patient engagement on the course and seriousness of cancer. It shows the greatest deviation in decision-making desires and emphasizes its crucial role in HIS customization and cancer patient decisional authority. The outcomes show a range of decision-making preferences, with differences depending on the type of cancer and the demographics. The findings emphasize the necessity of customized HIS that promotes knowledgeable, patient-focused treatment. HIS can authorize patients, improve oncology treatment generally, and enable provider collaboration by combining real-time data access and predictive analytics.
The investigation placed its focus on specific decision-making domains, which did not sufficiently cover the whole range of patient options, thus becoming one of its major limitations. The evaluation does not include an investigation of extended patient outcome changes brought by HIS and neglects possible emotional and psychological variables affecting decision-making. The components did not explore patient healthcare understanding to its deepest levels. Further investigation might be to examine customized HIS effects on patient outcomes during extended periods while factoring psychological judgment elements and analyzing the use of artificial intelligence technology in individualized cancer healthcare.
BIBLIOGRAPHIC REFERENCES
1. Allen LA, Venechuk G, McIlvennan CK, Page RL, Knoepke CE, Helmkamp LJ, Khazanie P, Peterson PN, Pierce K, Harger G, Thompson JS. An electronically delivered patient-activation tool for intensification of medications for chronic heart failure with reduced ejection fraction: the EPIC-HF trial. Circulation. 2021 Feb 2;143(5):427-37. https://doi.org/10.1038/s41436-019-0702-z
2. Treffers T, Putora PM. Emotions as social information in shared decision-making in oncology. Oncology. 2020 Jun 17;98(6):430-7. https://doi.org/10.1159/000505341
3. Tricou C, Yennu S, Ruer M, Bruera E, Filbet M. Decisional control preferences of patients with advanced cancer receiving palliative care. Palliative & supportive care. 2018 Oct;16(5):544-51. https://doi.org/10.1017/S1478951517000803
4. Dutta O, Lall P, Patinadan PV, Car J, Low CK, Tan WS, Ho AH. Patient autonomy and participation in end-of-life decision-making: An interpretive-systemic focus group study on perspectives of Asian healthcare professionals. Palliative & supportive care. 2020 Aug;18(4):425-30. https://doi.org/10.1017/S1478951519000865
5. Rundo L, Pirrone R, Vitabile S, Sala E, Gambino O. Recent advances of HCI in decision-making tasks for optimized clinical workflows and precision medicine. Journal of biomedical informatics. 2020 Aug 1;108:103479. https://doi.org/10.1016/j.jbi.2020.103479
6. Ankolekar A, Vanneste BG, Bloemen-van Gurp E, van Roermund JG, van Limbergen EJ, van de Beek K, Marcelissen T, Zambon V, Oelke M, Dekker A, Roumen C. Development and validation of a patient decision aid for prostate Cancer therapy: from paternalistic towards participative shared decision making. BMC medical informatics and decision making. 2019 Dec;19:1-1. https://doi.org/10.1186/s12911-019-0862-4
7. Aljabri D, Dumitrascu A, Burton MC, White L, Khan M, Xirasagar S, Horner R, Naessens J. Patient portal adoption and use by hospitalized cancer patients: a retrospective study of its impact on adverse events, utilization, and patient satisfaction. BMC medical informatics and decision making. 2018 Dec;18:1-2. https://doi.org/10.1186/s12911-018-0644-4
8. Agarwal R, Epstein AS. Advanced care planning and end-of-life decision-making for patients with cancer. In Seminars in oncology nursing 2018 Aug 1 (Vol. 34, No. 3, pp. 316-326). WB Saunders. https://doi.org/10.1016/j.soncn.2018.06.012
9. Perestelo-Perez L, Rivero-Santana A, Torres-Castaño A, Ramos-Garcia V, Alvarez-Perez Y, Gonzalez-Hernandez N, Buron A, Pignone M, Serrano-Aguilar P. Effectiveness of a decision aid for promoting colorectal cancer screening in Spain: a randomized trial. BMC Medical Informatics and Decision Making. 2019 Dec;19:1-9. https://doi.org/10.1186/s12911-019-0739-6
10. Wilson LS, Blonquist TM, Hong F, Halpenny B, Wolpin S, Chang P, Filson CP, Master VA, Sanda MG, Chien GW, Jones RA. Assigning value to preparation for prostate cancer decision making: a willingness to pay analysis. BMC medical informatics and decision making. 2019 Dec;19:1-9. https://doi.org/10.1186/s12911-018-0725-4
11. van Dams R, Grogan T, Lee P, Punglia R, Raldow A. Impact of health-related quality of life and prediagnosis risk of major depressive disorder on treatment choice for stage I lung cancer. JCO Clinical Cancer Informatics. 2019 Sep;1:1-8. https://doi.org/10.1200/CCI.19.00072
12. Filson CP, Hong F, Xiong N, Pozzar R, Halpenny B, Berry DL. Decision support for men with prostate cancer: concordance between treatment choice and tumor risk. Cancer. 2021 Jan 15;127(2):2038. https://doi.org/10.1002/cncr.33241
13. Fagerlin A, Holmes-Rovner M, Hofer TP, Rovner D, Alexander SC, Knight SJ, Ling BS, A. Tulsky J, Wei JT, Hafez K, Kahn VC. Head to head randomized trial of two decision aids for prostate cancer. BMC Medical Informatics and Decision Making. 2021 May 12;21(1):154. https://doi.org/10.1186/s12911-021-01505-x
14. Hlubocky FJ, Sher TG, Cella D, Wroblewski KE, Peppercorn J, Daugherty CK. Anxiety shapes expectations of therapeutic benefit in phase I trials for patients with advanced cancer and spousal caregivers. JCO oncology practice. 2021 Feb;17(2):e101-10. https://doi.org/10.1200/OP.20.00646
15. Bombard Y, Clausen M, Shickh S, Mighton C, Casalino S, Kim TH, Muir SM, Carlsson L, Baxter N, Scheer A, Elser C. Effectiveness of the Genomics ADvISER decision aid for the selection of secondary findings from genomic sequencing: a randomized clinical trial. Genetics in Medicine. 2020 Apr;22(4):727-35. https://doi.org/10.1038/s41436-019-0702-z
FINANCING
No financing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Data curation: Soumya Surath Panda, Manashree Mane, Sourabh Kumar Singh.
Methodology: Soumya Surath Panda, Manashree Mane, Sourabh Kumar Singh.
Software: Soumya Surath Panda, Manashree Mane, Sourabh Kumar Singh.
Drafting - original draft: Soumya Surath Panda, Manashree Mane, Sourabh Kumar Singh.
Writing - proofreading and editing: Soumya Surath Panda, Manashree Mane, Sourabh Kumar Singh.