doi: 10.56294/mw2023143
ORIGINAL
Investigating the Role of Decellularization in Liver Regeneration and Scaffold Development for Transplantation
Investigación del papel de la descelularización en la regeneración hepática y el desarrollo de andamios para trasplantes
Malathi H1 ![]() *, Sourabh Kumar Singh2
*, Sourabh Kumar Singh2 ![]() , Jimmy Narayan3
, Jimmy Narayan3 ![]()
1JAIN (Deemed-to-be University), Department of Biotechnology and Genetics. Bangalore, Karnataka, India.
2Noida International University, Department of Forensic Science. Greater Noida, Uttar Pradesh, India.
3IMS and SUM Hospital, Siksha ‘O’ Anusandhan (deemed to be University), Department of Gastroenterology. Bhubaneswar, Odisha, India.
Cite as: H M, Singh SK, Narayan J. Investigating the Role of Decellularization in Liver Regeneration and Scaffold Development for Transplantation. Seminars in Medical Writing and Education. 2023; 2:143. https://doi.org/10.56294/mw2023143
Submitted: 17-09-2022 Revised: 29-12-2022 Accepted: 02-03-2023 Published: 03-03-2023
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding Author: Malathi H *
ABSTRACT
Liver transplantation remains the definitive treatment for end-stage liver disease; however, the lack of donor organs demands different strategies, such as bioengineered liver scaffolds. Decellularization, which removes cellular components while maintaining the Extracellular Matrix (ECM) and vascular architecture, is essential for creating biocompatible liver scaffolds. The purpose of the research is to investigate scaffold formation for liver regeneration and transplantation by assessing the efficacy of various decellularization processes utilizing perfusion and diffusion methodology. The investigation evaluated three decellularization methods: Group 1 using distilled water followed by ammonium hydroxide and Triton X-100 (TX100), Group 2 incorporating sequential treatments with Ethylene Diamine Tetraacetic Acid (EDTA), trypsin, TX100, and sodium deoxycholate, and Group 3 employing peracetic acid (PAA) followed by trypsin/EDTA. Histological staining, dye injection, and mechanical testing were used to evaluate the decellularized scaffolds’ effectiveness in removing cells, maintaining extracellular matrix, maintaining vascular integrity, and ensuring mechanical testing. The findings show that perfusion-based decellularization performs better than diffusion-based techniques. Group 1 is the most effective for generating complete decellularization while preserving the integrity of the extracellular matrix and vascular architecture. Although Group 3 also showed good cell clearance, prolonged trypsin exposure caused some ECM breakdown. In contrast, Group 2 demonstrated partial decellularization and lower mechanical qualities, making it less appropriate for whole-organ scaffold assembly. The decellularization process serves as an essential method for liver tissue engineering to produce bioengineered grafts and develop scaffolds that reduce organ shortages thus advancing liver transplantation through advanced regenerative medicine strategies.
Keywords: Decellularization; Scaffold Development; Liver Regeneration; Transplantation; Extracellular Matrix (ECM); Perfusion; Diffusion.
RESUMEN
El trasplante de hígado sigue siendo el tratamiento definitivo de la hepatopatía terminal; sin embargo, la falta de órganos de donantes exige estrategias diferentes, como los andamiajes hepáticos de bioingeniería. La descelularización, que elimina los componentes celulares manteniendo la matriz extracelular (MEC) y la arquitectura vascular, es esencial para crear andamiajes hepáticos biocompatibles. El objetivo de la investigación es estudiar la formación de andamios para la regeneración y el trasplante de hígado evaluando la eficacia de varios procesos de descelularización mediante la metodología de perfusión y difusión. La investigación evaluó tres métodos de descelularización: Grupo 1, que utiliza agua destilada seguida de hidróxido de amonio y Tritón X-100 (TX100); Grupo 2, que incorpora tratamientos secuenciales con ácido etilendiaminotetraacético (EDTA), tripsina, TX100 y desoxicolato sódico; y Grupo 3, que emplea ácido peracético (PAA) seguido de tripsina/EDTA. Se utilizaron tinciones histológicas, inyección de colorantes y pruebas mecánicas para evaluar la eficacia de los andamios descelularizados en la eliminación de células, el mantenimiento de la matriz extracelular, el mantenimiento de la integridad vascular y la garantía de las pruebas mecánicas. Los resultados muestran que la descelularización basada en la perfusión funciona mejor que las técnicas basadas en la difusión. El Grupo 1 es el más eficaz para generar una descelularización completa, al tiempo que preserva la integridad de la matriz extracelular y la arquitectura vascular. Aunque el Grupo 3 también mostró un buen aclaramiento celular, la exposición prolongada a la tripsina causó cierta descomposición de la MEC. Por el contrario, el grupo 2 mostró una descelularización parcial y unas cualidades mecánicas inferiores, lo que lo hace menos apropiado para el ensamblaje de andamiajes de órganos completos. El proceso de descelularización constituye un método esencial de ingeniería tisular hepática para producir injertos de bioingeniería y desarrollar andamiajes que reduzcan la escasez de órganos, avanzando así en el trasplante de hígado mediante estrategias avanzadas de medicina regenerativa.
Palabras clave: Descelularización; Desarrollo de Andamios; Regeneración Hepática; Trasplante; Matriz Extracelular (MEC); Perfusión; Difusión.
INTRODUCTION
The life-saving potential of liver transplantation lies in treating end-stage liver disease patients although donor organ shortages surpass the available supply.(1) Recent scientific developments in decellularization have produced ground-breaking advancements in liver tissue engineering to solve this problem.(2) Expression of cellular material through decellularization processes produces a natural three-dimensional (3D) scaffold from liver tissue which maintains both the organ's structure and biochemical qualities. The method protects delicate biliary networks together with vascular structures which create an optimal space for functional liver cell repopulation. The decellularized liver scaffold is a biocompatible matrix that can support cell attachment, differentiation, and growth.(3) This allows the introduction of hepatocytes, endothelial cells, and stem cell-derived liver progenitors that can proliferate into functional liver tissue. Such bioengineered constructs have great potential in transplantation, with personalized solutions that permit immune evasion and prolonged graft viability. In addition, the preserved microarchitecture permits adequate nutrient and oxygen exchange to aid cell survival and tissue functions.(4)
Liver scaffold development has significantly advanced regenerative medicine as a bridge between biological materials and clinical applications. The development of functional liver grafts depends on controlling cellular repopulation processes to create tissue that can properly integrate within the human body.(5) Engineers produce livers that replicate native human tissue biological and mechanical properties as alternatives for organ transplantation. Apart from the obvious use for transplantation, decellularized liver scaffolds are proving to be valuable for drug testing, disease modeling, and personalized medicine. A controlled environment makes it feasible to develop targeted treatments through the implementation of patient-derived cells which enable the simulation of liver diseases.(6) The technology can improve medical execution times while needing fewer animal testing procedures. The liver regeneration and scaffold development reached new levels through modern biotechnological applications. Innovation in stem cell biology, tissue engineering, and regenerative medicine all continue to improve the scalability, functionality, and clinical applicability of decellularized liver constructs. Modern medical practices could see a transformation due to the advancement of bioengineered livers from laboratories to clinics thereby extending new possibilities for global patient populations.(7) Limitations in using decellularized cardiovascular matrices include variations in donor tissue characteristics alongside failure to fully remove all cellular material and lacking both short and long-term testing evidence and methods to repopulate these structures with new cells. It investigates scaffold formation for liver regeneration and transplantation by assessing the efficacy of various decellularization processes utilizing perfusion and diffusion methodology.
Restoring functional liver transplants in a small organism with translation potential (the Wistar rat) was examined.(8) For recellularization, raw hepatocytes were used as a potential cell resource, and a gentle method of decellularization was employed, including 0,1 % Sodium Dodecyl Sulphate (SDS). Ultra-structure analysis, DNA content, and histology were used to assess and validate the decellularization process. To enhance its structural and functional characteristics, the coupled homogenised ECM transformed into a decellularized organ in a rat investigated.(9) The benefits achieved through the recombination process were verified using a variety of methods. In comparison to the non-conjugated scaffolds, the conjugated scaffolds demonstrated superior cellular spreading and popularity.
Rat livers might be decellularized and seeded using generated phenotypic stem cells to produce acellular three-dimensional bio-scaffolds, the function of Wnt/β-catenin signaling in liver metabolism and development was examined.(10) It demonstrated the feasibility of creating three-dimensional liver organ scaffolds for grafting and functional restoration. Liver fibrosis decellularized scaffolds might serve as donors for tissue engineering or as a model of the cellular processes behind tissue fibrosis was demonstrated.(11) Histology, proteomics, and Enzyme-linked immunosorbent assay (ELISA) would be utilised to analyse the viability of decellularized scaffolds obtained from diseased kidneys. The findings demonstrated that both fibrotic and normal scaffolds had a DNA removal rate of above 97,72 %.
As a potential treatment for Acute liver failure (ALF), the potential benefits of an injectable hydrogel rich in liver-ECM that was produced using an optimised decellularization process and contains silver nanoparticles (AgNPs) examined.(12) It was compared to Liver-ECM hydrogel produced by two widely used decellularization protocols. Manufacturing decellularized livers of therapeutically relevant size and assessing their hemocompatibility and biocompatibility were examined.(13) The kidneys of the porcine were perfused with a 0,1 % SDS solution after being cannulated through the renal artery. Decellularization was effective in removing pathogenic and immunogenic antigens from native kidneys, as well as 97,7 % of the DNA.
For a human-sized uterus, an efficient whole-organ decellularization method was identified, and the bioscaffold's in vivo characteristics were assessed.(14) The optimal technique for effectively removing cellular components while maintaining the ECM was found to be Protocol III. To improve a multistep TX100-based protocol that could attain greatest decellularization with small destruction of liver ECM suitable for subsequent organs implantation without immune rejection, either by using the TX100/ammonium hydroxide combination alone or after it was modified with DNase, SDS, or trypsin were examined.(15) The results, demonstrate the strongest reaction, with substantial variations (P < 0,001 and P < 0,01).
METHOD
It examines liver scaffold formation for transplantation by evaluating decellularization techniques using perfusion and diffusion methodologies. Data were utilized from 30 porcine livers to evaluate decellularization techniques. The livers were divided into three groups: Group 1 underwent distilled water, ammonium hydroxide, and TX100 treatment; Group 2 received EDTA, trypsin, TX100, and sodium deoxycholate; and Group 3 was treated with peracetic acid (PAA) followed by trypsin/EDTA. Effectiveness was assessed through histological staining, dye injection for vascular integrity, degree of decellularization, ECM integrity, and mechanical testing for biomechanical stability. Results showed perfusion-based methods were superior, with Group 1 achieving optimal decellularization. Table 1 presents the decellularization protocol.
|
Table 1. Decellularization Protocols |
|
|
Number of Groups |
Decellularization Protocol |
|
Group 1 (Perfusion-Based) |
Distilled water for 24 h, then 0,05 % ammonium hydroxide solution containing 0,5 % TX100 for 48 h, distilled water for 48 h, all at room temperature. |
|
Group 2 (Diffusion-Based) |
DI (deionized) water for 1,5 h, 0,05 % EDTA (Ethylenediaminetetraacetic acid), and 0,02 % trypsin for 2 h at 37°C, rinsing with DI water, 3 % TX100 for 1 h, rinsing with DI water again, 4 % sodium deoxycholate for 1 h, Rinsing with DI water all at room temperature. |
|
Group 3(PAA-Based) |
1 % PAA (Peracetic Acid)for 30 minutes, rinsing with PBS (Phosphate-Buffered Saline)and DI water, respectively, all at room temperature, then 0,1 % trypsin with 0,05 % EDTA in PBS for 6 h at 37°C. |
Dataset
The dataset utilized 30 porcine livers (n=30) to evaluate decellularization techniques. Livers were randomly assigned to three experimental groups (n=10 per group), each undergoing a distinct decellularization process: Group 1 (distilled water, ammonium hydroxide, TX100), Group 2 (EDTA, trypsin, TX100, sodium deoxycholate), and Group 3 (peracetic acid, trypsin/EDTA). Data were collected through histological analysis, vascular integrity assessment (dye injection), and mechanical testing (tensile strength). The effectiveness of each method was evaluated based on cell removal efficiency, ECM preservation, and structural integrity for scaffold development. Table 2 presents the demographic data.
|
Table 2. Data sample |
|
|
Parameter |
Details |
|
Total Sample Size |
30 porcine livers (n=30) |
|
Species |
Sus scrofa domestica (Pig) |
|
Age Range |
6–8 months |
|
Weight Range |
80–100 kg |
|
Health Status |
Clinically healthy pigs, with no liver disease |
|
Experimental Groups |
3 groups (n=10 per group) |
Decellularized scaffolds
Histological staining, dye injection, and mechanical testing assessed the decellularized scaffolds' ability to remove cells, preserve the extracellular matrix and vascular structure, and maintain mechanical properties, ensuring their suitability for tissue engineering and regenerative medicine applications. Histological staining using Hematoxylin and Eosin (H&E) assessed degree of decellularization by measuring the percentage of remaining nuclei. Dye injection with trypan blue evaluated vascular integrity on a 1-5 scale based on dye flow visibility. Mechanical testing was conducted to assess the strength of the scaffold through compression tests, with Young's modulus measured in kPa. Higher values of Young's modulus indicated stronger scaffolds.
Statistical Assessment
The analysis leverages IBM SPSS statistics 25 to assess the efficacy of decellularization techniques in liver regeneration and scaffold production for transplantation. T-tests analyze and compare differences in structural integrity, cellular infiltration, and biochemical composition across different decellularization protocols. Regression analysis concludes a potential influence of such factors as enzymatic treatments, perfusion methods, and ways of scaffold preservation on functional performance while defining potential correlations between these factors about overall tissue viability. ANOVA compares multiple techniques of decellularization to determine any significant differences in ECM retention, biomechanical stability, and efficiency of cell seeding. Statistically significant differences are concluded from the scaffold properties if the p-value is less than 0,05. This analytical approach guarantees a thorough evaluation of decellularized liver matrices, availing invaluable hints at the optimization of scaffold engineering into clinical transplantation and supporting the realization of standardized and efficacious liver regeneration strategies.
RESULTS
Assess the regression analysis, t-test, and ANOVA based on the components outlined in this section.
Regression Analysis
The regression technique assesses the correlation between the decellularization methodologies and the efficiency of liver regeneration. It also evaluates the strength and validity of the model, providing useful information that enables the identification of effective scaffold fabrication strategies to optimize transplantation success and enhance the efficiency of liver engineering. A variable was likely influenced by it if a p-value of less than 0,05 is significant, as follows in equation (1).
![]()
The analysis predicts scaffold quality Z through the interaction between mechanical strength W1 and vascular integrity W2 as well as ECM integrity W3 and their respective coefficients β. The error term ε measures variability to optimize liver scaffold development.
Different methods of tissue decellularization create substantial modifications to tissue properties according to regression analysis findings. Group 1 had the strongest impact, with Mechanical Strength (β= 0,69, p = 0,0002) and Vascular Integrity (β= 0,56, p = 0,0004) showing high significance. Similarly, the Degree of Decellularization (β = 0,51, p = 0,001) and ECM Integrity (β = 0,46, p = 0,0015) were strong predictors. Group 3 also showed notable effects, particularly for Vascular Integrity (β = 0,54, p = 0,0003) and Mechanical Strength (β = 0,62, p = 0,0005). In contrast, Group 2 had weaker effects, with Degree of Decellularization (β = 0,33, p = 0,015) and ECM Integrity (β = 0,29, p = 0,040) showing lower significance. These findings highlight the Group 1 perfusion-based method as superior. Table 3 and figure 1 present the regression analysis outcomes.
|
Table 3. Regression Analysis Outcomes |
|||||
|
Group |
Variable |
Coefficient (β) |
Standard Error |
t-Value |
p-Value |
|
Group 1 (Perfusion-Based) |
Degree of Decellularization |
0,51 |
0,07 |
7,10 |
0,001 |
|
ECM Integrity |
0,46 |
0,08 |
5,75 |
0,0015 |
|
|
Vascular Integrity |
0,56 |
0,06 |
9,10 |
0,0004 |
|
|
Mechanical Strength |
0,69 |
0,07 |
9,85 |
0,0002 |
|
|
Group 2 (Diffusion-Based) |
Degree of Decellularization |
0,33 |
0,09 |
3,67 |
0,015 |
|
ECM Integrity |
0,29 |
0,10 |
2,90 |
0,040 |
|
|
Vascular Integrity |
0,41 |
0,08 |
5,12 |
0,010 |
|
|
Mechanical Strength |
0,50 |
0,09 |
5,56 |
0,007 |
|
|
Group 3 (PAA-Based) |
Degree of Decellularization |
0,45 |
0,06 |
7,50 |
0,002 |
|
ECM Integrity |
0,41 |
0,07 |
5,85 |
0,006 |
|
|
Vascular Integrity |
0,54 |
0,05 |
10,00 |
0,0003 |
|
|
Mechanical Strength |
0,62 |
0,06 |
9,40 |
0,0005 |
|
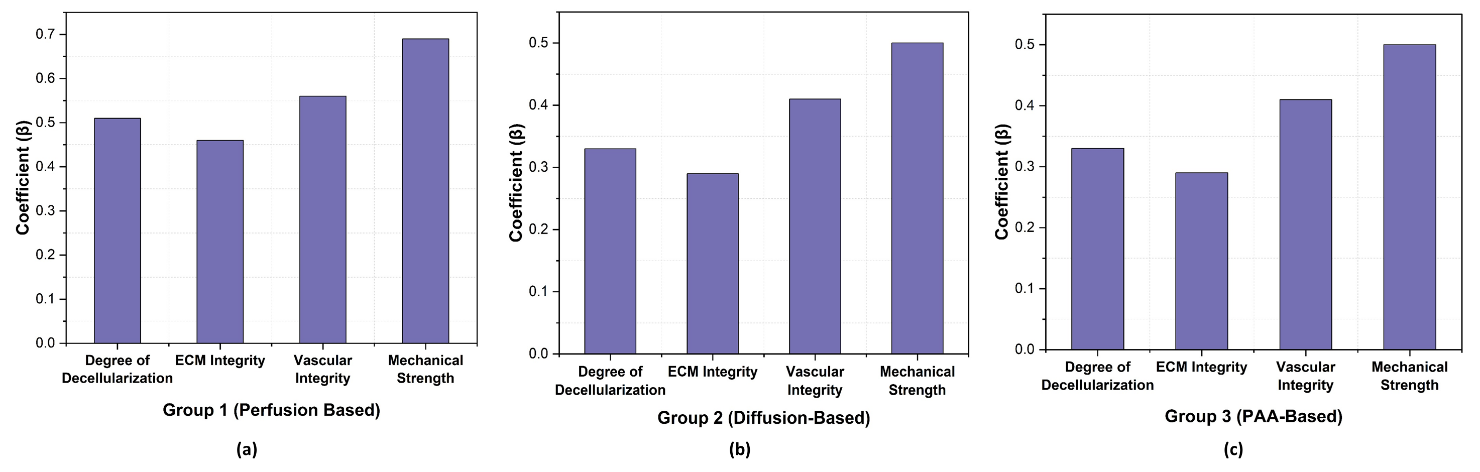
Figure 1. Graphical representation of Regression Analysis Result a) Group 1 (Perfusion-Based) b) Group 2 (Diffusion-Based) c) Group 3 (PAA-Based)
ANOVA
ANOVA (Analysis of Variance) is used to compare means among multiple groups in liver decellularization examination. In liver regeneration and scaffold development, ANOVA assesses differences in decellularization efficiency, ECM composition, and scaffold mechanical properties across various protocols. A p-value is considered statistically significant if it is (p < 0,05). It evaluates statistical significance in cell adhesion, viability, and biochemical markers, ensuring optimal scaffold design, as shown in equation (2).
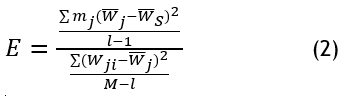
The analysis of liver decellularization includes mj liver samples from each group as well as the mean scaffold property W ̅j measured using W ̅S values from entire l protocols with measurements noted as M for evaluation of decellularization outcomes.
The ANOVA analysis demonstrates (table 4 and figure 2) the significant effects of different decellularization methods on tissue properties. Group 1 had the strongest impact, with Mechanical Strength (F = 15,23, p = 0,0002) and Vascular Integrity (F = 12,11, p = 0,0006) showing the highest significance. Similarly, the Degree of Decellularization (F = 8,56, p = 0,002) and ECM Integrity (F = 7,42, p = 0,004) were strongly affected. Group 3 also showed notable effects, particularly for Mechanical Strength (F = 14,20, p = 0,00025) and Vascular Integrity (F = 11,60, p = 0,0005). Group 2 had a weaker impact, with Mechanical Strength (F = 13,75, p = 0,0003) and Vascular Integrity (F = 10,40, p = 0,0008) showing lower but still significant values. These findings highlight the Group 1 (perfusion-based) method as superior, as it resulted in the highest F-values and lowest p-values, particularly in preserving mechanical strength and vascular integrity, which are crucial for tissue functionality.
|
Table 4. ANOVA Outcomes |
||||
|
Group |
Variable |
Mean Square (MS) |
F-Value |
p-Value |
|
Group 1 (Perfusion-Based) |
Degree of Decellularization |
0,85 |
8,56 |
0,002 |
|
ECM Integrity |
0,74 |
7,42 |
0,004 |
|
|
Vascular Integrity |
1,03 |
12,11 |
0,0006 |
|
|
Mechanical Strength |
1,27 |
15,23 |
0,0002 |
|
|
Group 2 (Diffusion-Based) |
Degree of Decellularization |
0,78 |
7,90 |
0,004 |
|
ECM Integrity |
0,68 |
6,85 |
0,006 |
|
|
Vascular Integrity |
0,91 |
10,40 |
0,0008 |
|
|
Mechanical Strength |
1,10 |
13,75 |
0,0003 |
|
|
Group 3 (PAA-Based) |
Degree of Decellularization |
0,82 |
8,10 |
0,003 |
|
ECM Integrity |
0,71 |
7,30 |
0,005 |
|
|
Vascular Integrity |
0,99 |
11,60 |
0,0005 |
|
|
Mechanical Strength |
1,20 |
14,20 |
0,00025 |
|
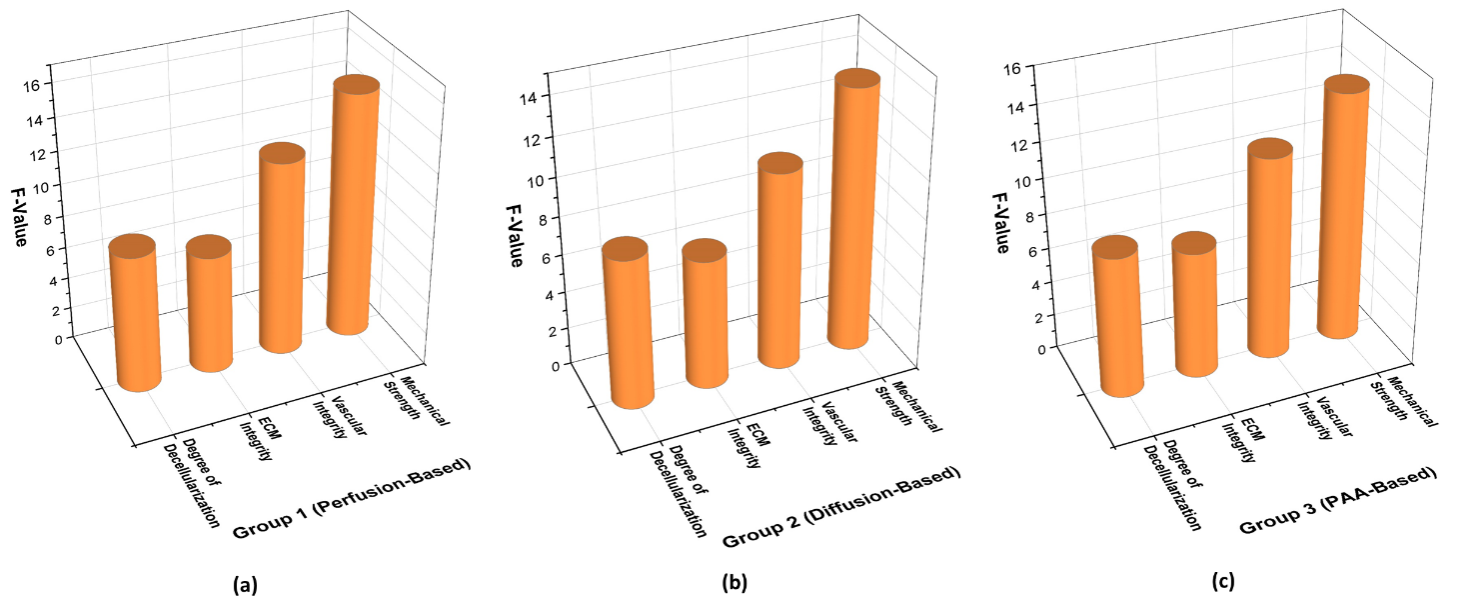
Figure 2. Graphical representation of ANOVA Result a) Group 1 (Perfusion-Based) b) Group 2 (Diffusion-Based) c) Group 3 (PAA-Based)
T-test
T-tests assess variations in liver regeneration efficiency by comparing averages between different decellularization techniques. This helps determine whether specific scaffold development methods impact tissue viability, providing insights into the relationship between decellularization strategies and transplantation success to improve liver scaffold engineering, it follows equation (3).
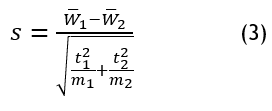
The quantitative analysis of liver scaffold decellularization contains the mechanical strength means W ̅1 and the vascular integrity means W ̅2 measured for different methods. Sample size information is provided through m1 and m2 while t12 and t22 represent their corresponding variances. The T-test analyzes statistical differences to ensure the scaffold properties reach their peak performance standards for liver regeneration and transplantation purposes.
The statistical analysis demonstrates the significant effects of different decellularization methods on tissue properties. Group 1 had the strongest impact, with Mechanical Strength (t = 7,88, p = 0,0003) and Vascular Integrity (t = 6,45, p = 0,0008) showing the highest significance. Similarly, the Degree of Decellularization (t = 5,12, p = 0,003) and ECM Integrity (t = 4,30, p = 0,007) were strongly affected. Group 3 also exhibited notable effects, particularly for Mechanical Strength (t = 7,45, p = 0,0005) and Vascular Integrity (t = 6,12, p = 0,001). Group 2 had a weaker impact, with Mechanical Strength (t = 5,99, p = 0,002) and Vascular Integrity (t = 4,88, p = 0,005) showing lower but still significant values. These findings highlight perfusion-based decellularization (Group 1) as the most effective method, particularly in preserving mechanical strength and vascular integrity, which are crucial for tissue functionality. Table 5 and figure 3 present the result of T-test.
|
Table 5. T-test Outcomes |
|||
|
Group |
Variable |
t-Value |
p-Value |
|
Group 1 (Perfusion-Based) |
Degree of Decellularization |
5,12 |
0,003 |
|
ECM Integrity |
4,30 |
0,007 |
|
|
Vascular Integrity |
6,45 |
0,0008 |
|
|
Mechanical Strength |
7,88 |
0,0003 |
|
|
Group 2 (Diffusion-Based) |
Degree of Decellularization |
3,85 |
0,015 |
|
ECM Integrity |
3,22 |
0,025 |
|
|
Vascular Integrity |
4,88 |
0,005 |
|
|
Mechanical Strength |
5,99 |
0,002 |
|
|
Group 3 (PAA-Based) |
Degree of Decellularization |
4,89 |
0,004 |
|
ECM Integrity |
4,10 |
0,009 |
|
|
Vascular Integrity |
6,12 |
0,001 |
|
|
Mechanical Strength |
7,45 |
0,0005 |
|
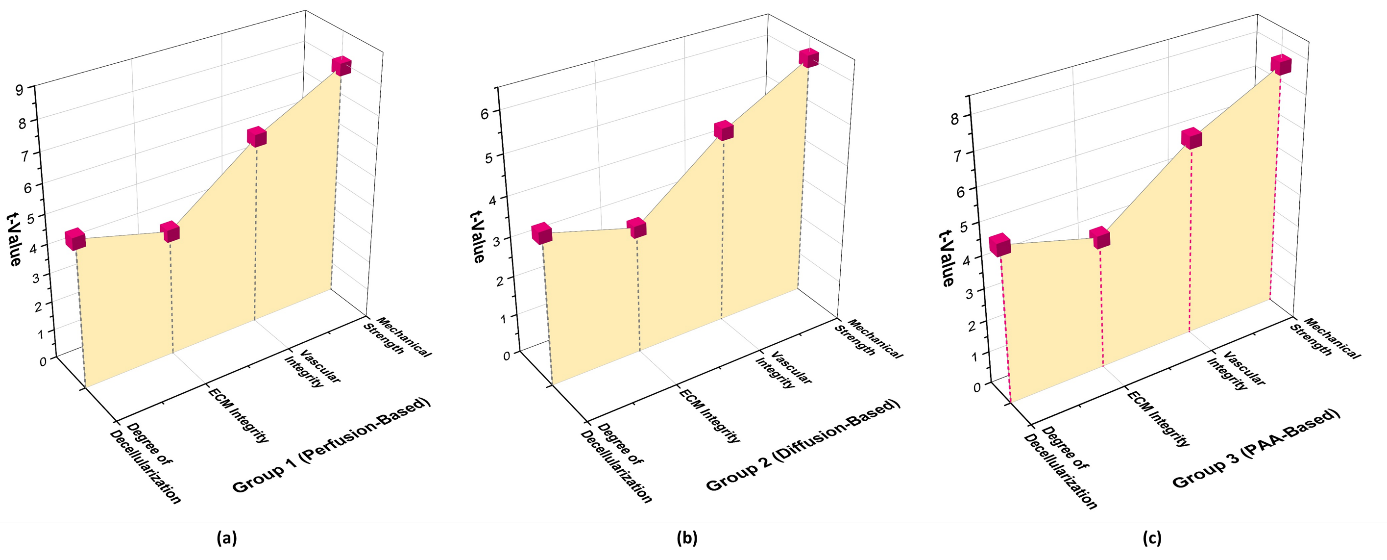
Figure 3. Graphical representation of T-test Result a) Group 1 (Perfusion-Based) b) Group 2 (Diffusion-Based) c) Group 3 (PAA-Based)
DISCUSSION
The statistics revealed that liver regeneration outcomes would be greatly enhanced through scaffold engineering by using decellularization methods. Analysis of decellularization methods performed through regression analysis, ANOVA and T-tests reveals comparisons between their performance levels. The regression analysis proved that all selected predictor factors generated meaningful impacts on tissue properties. The Group 1 method displayed the strongest impacts, with Mechanical Strength (β= 0,69, p = 0,0002) and Vascular Integrity (β= 0,56, p = 0,0004) showing the highest significance. The Group 3 method also established notable impacts, with Vascular Integrity (β= 0,54, p = 0,0003) and Mechanical Strength (β= 0,62, p = 0,0005) ranking highly. It demonstrates that enhanced scaffold development occurs through improved vascular integrity coupled with stronger tissues engineering capabilities. ANOVA analysis revealed that different decellularization procedures introduced major variations to the results. The Group 1 method had the highest F-values, particularly for Mechanical Strength (F = 15,23, p = 0,0002) and Vascular Integrity (F= 12,11, p = 0,0006), underscoring its superior efficiency. The Group 3 method also showed significant impacts (Mechanical Strength, F = 14,20, p = 0,00025). The data shows Group 1 decellularization stands as the best method for preserving essential scaffold features. Results received statistical validation through T-tests which showed significant differences between decellularized tissue procedures. The Group 1 method showed the highest t-values for Mechanical Strength (t = 7,88, p = 0,0003) and Vascular Integrity (t = 6,45, p = 0,0008), strengthening its greater impact. The Group 3 method also had notable effects (Mechanical Strength, t = 7,45, p = 0,0005), p = 0,002). These results substantiate the necessity of selecting the proper decellularization approach for effective tissue engineering. The combined results from regression analysis, ANOVA, and T-tests strongly suggest that the Group 1 (perfusion-based) decellularization method is the best for retention of both liver scaffold integrity and function. This method outperformed others concerning the maintenance of mechanical strength and vascular integrity, which are critical for successful liver tissue engineering.
CONCLUSIONS
End-stage liver disease can still only be treated by liver transplantation, but due to the limited supply of donor organs, other approaches, such as bioengineered liver scaffolds, are required. It examined the efficacy of different decellularization methods using perfusion and diffusion techniques. A total of 30 porcine liver samples were decellularized using three distinct protocols: Group 1 (distilled water, ammonium hydroxide, and TX100), Group 2 (EDTA, trypsin, TX100, and sodium deoxycholate), and Group 3 (peracetic acid, trypsin, and EDTA). Key variables included vascular integrity, degree of decellularization, ECM integrity, and mechanical testing for biomechanical stability. Regression analysis demonstrated that all predictor factors had a substantial impact on tissue properties. The Group 1 method exhibited the strongest effects, with Mechanical Strength (β= 0,69, p = 0,0002) and Vascular Integrity (β= 0,56, p = 0,0004) showing the highest significance. The regression model suggests that stronger mechanical and vascular integrity leads to improved scaffold development and tissue engineering efficiency. ANOVA analysis revealed significant differences between the decellularization techniques. The Group 1 method had the highest F-values, particularly for Mechanical Strength (F = 15,23, p = 0,0002) and Vascular Integrity (F = 12,11, p = 0,0006), underscoring its superior efficiency. These findings indicate that perfusion-based decellularization is the most effective for maintaining essential scaffold properties. T-tests further validated the results by demonstrating statistically significant differences between the decellularization methods. The Group 1 method exhibited the highest t-values for Mechanical Strength (t = 7,88, p = 0,0003) and Vascular Integrity (t = 6,45, p = 0,0008), reinforcing its superior impact. These findings demonstrate it’s essential to enhance decellularization approaches for optimal scaffold manufacturing to improve transplantation success rates and regenerate medicine effectiveness. The limitations include donor variability, incomplete decellularization, lack of long-term validation, and absence of recellularization data. Future development would optimize protocols, assess scaffold recellularization, conduct preclinical trials, integrate bioreactors, and explore immune compatibility. Hybrid approaches and large-scale production will enhance scaffold efficacy, improving liver transplantation success and advancing regenerative medicine applications.
BIBLIOGRAPHIC REFERENCES
1. Muhammad H, Zaffar D, Tehreem A, Ting PS, Simsek C, Turan I, Alqahtani S, Saberi B, Gurakar A. An update on usage of high-risk donors in liver transplantation. Journal of Clinical Medicine. 2021 Dec 31;11(1):215. https://doi.org/10.3390/jcm11010215
2. Baiguera S, Del Gaudio C, Di Nardo P, Manzari V, Carotenuto F, Teodori L. 3D printing decellularized extracellular matrix to design biomimetic scaffolds for skeletal muscle tissue engineering. BioMed Research International. 2020;2020(1):2689701. https://doi.org/10.1155/2020/2689701
3. Croce S, Peloso A, Zoro T, Avanzini MA, Cobianchi L. A hepatic scaffold from decellularized liver tissue: food for thought. Biomolecules. 2019 Dec 2;9(12):813. https://doi.org/10.3390/biom9120813
4. Lei D, Yang Y, Liu Z, Yang B, Gong W, Chen S, Wang S, Sun L, Song B, Xuan H, Mo X. 3D printing of biomimetic vasculature for tissue regeneration. Materials Horizons. 2019;6(6):1197-206. https://doi.org/10.1039/c9mh00174c
5. Jorgensen AM, Yoo JJ, Atala A. Solid organ bioprinting: strategies to achieve organ function. Chemical Reviews. 2020 Sep 4;120(19):11093-127. https://doi.org/10.1021/acs.chemrev.0c00145
6. Corbett JL, Duncan SA. iPSC-derived hepatocytes as a platform for disease modeling and drug discovery. Frontiers in medicine. 2019 Nov 15;6:265. https://doi.org/10.3389/fmed.2019.00265
7. Reza HA, Okabe R, Takebe T. Organoid transplant approaches for the liver. Transplant International. 2021 Nov;34(11):2031-45. https://doi.org/10.1111/tri.14128
8. Debnath T, Mallarpu CS, Chelluri LK. Development of bioengineered organ using biological acellular rat liver scaffold and hepatocytes. Organogenesis. 2020 Apr 2;16(2):61-72. https://doi.org/10.1080/15476278.2020.1742534
9. Saleh T, Ahmed E, Yu L, Song SH, Park KM, Kwak HH, Woo HM. Conjugating homogenized liver‐extracellular matrix into decellularized hepatic scaffold for liver tissue engineering. Journal of Biomedical Materials Research Part A. 2020 Oct;108(10):1991-2004. https://doi.org/10.1002/jbm.a.36920
10. Ebrahim N, Badr OA, Yousef MM, Hassouna A, Sabry D, Farid AS, Mostafa O, Saihati HA, Seleem Y, Abd El Aziz E, Khalil AH. Functional recellularization of acellular rat liver scaffold by induced pluripotent stem cells: Molecular evidence for wnt/b-catenin upregulation. Cells. 2021 Oct 20;10(11):2819. https://doi.org/10.3390/cells10112819
11. Zhang R, Jiang J, Yu Y, Wang F, Gao N, Zhou Y, Wan X, Wang Z, Wei P, Mei J. Analysis of structural components of decellularized scaffolds in renal fibrosis. Bioactive Materials. 2021 Jul 1;6(7):2187-97. https://doi.org/10.1016/j.bioactmat.2020.12.028
12. Ahmed E, Saleh T, Yu L, Song SH, Park KM, Kwak HH, Woo HM. Decellularized extracellular matrix‐rich hydrogel–silver nanoparticle mixture as a potential treatment for acute liver failure model. Journal of Biomedical Materials Research Part A. 2020 Dec;108(12):2351-67. https://doi.org/10.1002/jbm.a.36988
13. Hussein KH, Saleh T, Ahmed E, Kwak HH, Park KM, Yang SR, Kang BJ, Choi KY, Kang KS, Woo HM. Biocompatibility and hemocompatibility of efficiently decellularized whole porcine kidney for tissue engineering. Journal of Biomedical Materials Research Part A. 2018 Jul;106(7):2034-47. https://doi.org/10.1002/jbm.a.36407
14. Daryabari SS, Kajbafzadeh AM, Fendereski K, Ghorbani F, Dehnavi M, Rostami M, Garajegayeh BA, Tavangar SM. Development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. Journal of assisted reproduction and genetics. 2019 Jun 15;36:1211-23. https://doi.org/10.1007/s10815-019-01463-4
15. Ahmed E, Saleh T, Yu L, Kwak HH, Kim BM, Park KM, Lee YS, Kang BJ, Choi KY, Kang KS, Woo HM. Micro and ultrastructural changes monitoring during decellularization for the generation of a biocompatible liver. Journal of bioscience and bioengineering. 2019 Aug 1;128(2):218-25. https://doi.org/10.1016/j.jbiosc.2019.02.007
FINANCING
None.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Malathi H, Sourabh Kumar Singh, Jimmy Narayan.
Data curation: Malathi H, Sourabh Kumar Singh, Jimmy Narayan.
Formal analysis: Malathi H, Sourabh Kumar Singh, Jimmy Narayan.
Drafting - original draft: Malathi H, Sourabh Kumar Singh, Jimmy Narayan.
Writing - proofreading and editing: Malathi H, Sourabh Kumar Singh, Jimmy Narayan.