doi: 10.56294/mw2023144
ORIGINAL
Research on Whole Organ Decellularization Protocols for Kidney Transplantation and Vascular Integrity Evaluation
Investigación sobre protocolos de descelularización de órganos enteros para trasplante renal y evaluación de la integridad vascular
Yogendra Bhati1 ![]() *, Biswaranjan Mohanty2
*, Biswaranjan Mohanty2 ![]() , Manashree Mane3
, Manashree Mane3 ![]()
1Noida International University, Department of Pharmacology. Greater Noida, Uttar Pradesh, India.
2IMS and SUM Hospital, Siksha ‘O’ Anusandhan (deemed to be University), Department of Nephrology. Bhubaneswar, Odisha, India.
3JAIN (Deemed-to-be University), Department of Forensic science. Bangalore, Karnataka, India.
Cite as: Bhati Y, Mohanty B, Mane M. Research on Whole Organ Decellularization Protocols for Kidney Transplantation and Vascular Integrity Evaluation. Seminars in Medical Writing and Education. 2023; 2:144. https://doi.org/10.56294/mw2023144
Submitted: 17-09-2022 Revised: 29-12-2022 Accepted: 02-03-2023 Published: 03-03-2023
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding Author: Yogendra Bhati *
ABSTRACT
The shortage of donor kidneys highlights the need for innovative approaches to transplantation. Whole-organ decellularization creates acellular scaffolds suitable for recellularization with recipient-derived cells, reducing the risk of immune rejection. A key challenge is preserving vascular integrity during decellularization to ensure organ functionality. This aims to compare three decellularization protocols, detergent-based, enzyme-based, and a combined approach, for their effectiveness in maintaining vascular integrity (VI), ECM retention, and perfusion capacity. Additionally, factors influencing VI are analyzed using statistical techniques. Kidneys were processed using the three protocols. VI was measured using parameters such as ECM retention percentage, vascular leakage rates, and perfusion capacity. Pearson correlation analysis determined correlations between parameters such as decellularization time and vascular outcomes. One-way ANOVA contrasted protocol performance, and logistic regression determined predictors of preservation of VI. There were significant differences in ECM retention between protocols. The detergent-based process showed superior ECM retention and reduced vascular leakage compared to other processes. Logistic regression found decellularization time and solution concentration were important predictors of vascular preservation. The detergent-based protocol better maintained VI and ECM retention, thus holding potential for future clinical use in kidney transplantation. Additional optimization might further improve scaffold quality and functional results.
Keywords: Whole-Organ Decellularization; Kidney Transplantation; Vascular Integrity; Extracellular Matrix (ECM) Retention.
RESUMEN
La escasez de riñones de donantes pone de manifiesto la necesidad de adoptar enfoques innovadores en materia de trasplantes. La descelularización de todo el órgano crea andamios acelulares adecuados para la recelularización con células derivadas del receptor, lo que reduce el riesgo de rechazo inmunitario. Un reto clave es preservar la integridad vascular durante la descelularización para garantizar la funcionalidad del órgano. El objetivo es comparar la eficacia de tres protocolos de descelularización, uno basado en detergentes, otro en enzimas y otro combinado, para mantener la integridad vascular (VI), la retención de ECM y la capacidad de perfusión. Además, se analizan mediante técnicas estadísticas los factores que influyen en la VI. Los riñones se procesaron utilizando los tres protocolos. La VI se midió mediante parámetros como el porcentaje de retención de ECM, las tasas de fuga vascular y la capacidad de perfusión. El análisis de correlación de Pearson determinó las correlaciones entre parámetros como el tiempo de descelularización y los resultados vasculares. El ANOVA de una vía contrastó el rendimiento del protocolo y la regresión logística determinó los factores predictivos de la preservación del VI. Hubo diferencias significativas en la retención de ECM entre protocolos. El proceso basado en detergente mostró una mayor retención de ECM y una menor fuga vascular en comparación con otros procesos. La regresión logística determinó que el tiempo de descelularización y la concentración de la solución eran factores predictivos importantes de la preservación vascular. El protocolo basado en detergente mantuvo mejor la retención de VI y ECM, por lo que tiene potencial para un futuro uso clínico en el trasplante de riñón. Una optimización adicional podría mejorar aún más la calidad del andamiaje y los resultados funcionales.
Palabras clave: Descelularización de Órgano Completo; Trasplante Renal; Integridad Vascular; Retención de la Matriz Extracelular (MEC).
INTRODUCTION
Organ decellularization is currently a ground-breaking technique in regenerative medicine, offering a substitute for organ shortages and reducing the risks of immunological rejection following kidney transplantation (KT). Decellularization is the process of extracting all cellular material from a donor kidney while leaving the extracellular matrix (ECM) intact, which acts as a structural scaffold for recellularization with patient-specific cells. Vascular integrity (VI) is a critical feature of this method because it preserves blood flow and the delivery of nutrients after transplantation. Perfusion-based decellularization is a common method, where the native vasculature of the kidney is used to perfuse detergents, like Triton X-100 and Sodium Dodecyl Sulfate (SDS), via the renal artery with the goal of cellular component removal and maintaining the ECM and vascular architecture, which is then analyzed using histological staining, scanning electron microscopy (SEM), and perfusion analyses to determine vascular integrity.(1) Figure 1 illustrates the function of kidney transplantation from donor organ to functional scaffold.
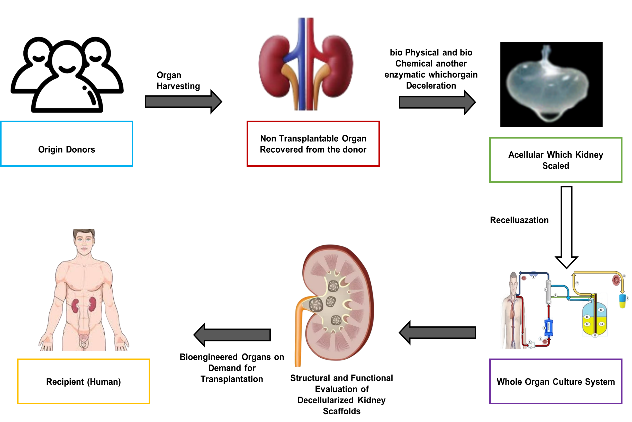
Figure 1. Kidney Transplantation: From Donor Organ to Functional Scaffold
The viability for decellularized kidneys serves as an essential requirement for successful recellularization and transplantation procedures. Research shows that the process of re-endothelialization through human pluripotent stem cell-derived endothelial cells (hiPSC-ECs) demonstrates positive results. In models, VEGF and Ang-1 treated scaffolds supported endothelial adhesion effectively and resulted in total re-endothelialization of glomerular and peritubular capillaries.(2) Furthermore, perfusion-based decellularization of human fetal kidneys has shown successful cell removal without loss of the ECM and vascular networks, which can be used for recellularization purposes. There are still many challenges in taking kidney decellularization from research in the lab to clinical use. Scaling up from animal models to human organs is one significant challenge because human kidneys possess more structural complexity and functional requirements.(3) Attaining full and even recellularization, especially in the microvasculature, is yet a major challenge. The origin of recellularizing cells is also a key issue, with patient-specific hiPSC-derived cells being promising to minimize immune rejection. To produce enough functional cells and integrating into the scaffold appropriately are areas that need more research. Moreover, the long-term functionality and survival of recellularized kidneys need to be evaluated to establish their success in transplantation.(4)
Cell removal offers a hopeful innovation in kidney transplantation through the use of a biocompatible scaffold that lowers the risk of immune rejection. Perfusion -based strategies need to be optimized so that efficient cell removal can be achieved without disrupting the extracellular matrix (ECM) or vascular network. The use of re-endothelialization strategies involving hiPSC-derived endothelial cells also further increases the viability and functionality of decellularized kidneys to better adapt them for future clinical use. Decellularization procedures, recellularization techniques, and addressing long-term problems with transplantation are presently the subject of research in progress, which are important milestones towards using this technology in the clinic successfully.(5) The goal of the research is to compare detergent-based, enzyme-based, and combined decellularization protocols for their effectiveness in preserving VI, ECM retention, and perfusion capacity, using statistical analysis techniques.
VI in decellularized kidney scaffolds was evaluated before and after cryostorage using a Micro-CT imaging method, which utilized contrast-enhanced Microfil infusion and X-ray imaging.(6) Whole rat kidneys were decellularized using Triton X-100 and DNase, then cryopreserved with various cryoprotectant solutions. Results confirmed structural alterations in vasculature post-freezing, but vitrification with VS83 preserved VI. A limitation was the challenge of fully preventing freezing damage, requiring further optimization for clinical applications. The preparation of clinically relevant decellularized kidneys and the assessment of their biocompatibility and hemocompatibility were investigated.(7) Porcine kidneys were decellularized using 0,1 % SDS perfusion via the renal artery. Histological staining, SEM, and contrast radiography confirmed cellular removal, ECM preservation, and intact vasculature. The scaffolds exhibited good hemocompatibility and did not induce an immune response. KT cells proliferated effectively within the scaffolds. A limitation was the susceptibility to enzymatic degradation, requiring further optimization for long-term stability in clinical applications.
Two whole-organ decellularization protocols for preserving ECM and VI in sheep kidneys were evaluated.(8) Sheep kidneys were decellularized using perfusion-based methods. Histopathology and CT angiography assessed decellularization and vasculature. Protocol 1 preserved ECM and vascular integrity better than Protocol 2. Post-transplant, Protocol 2 showed fluid extravasation, while Protocol 1 maintained structure. A limitation was the short-term monitoring, requiring further long-term validation. The impact of dynamic blood perfusion on cell removal in porcine kidney scaffolds was evaluated.(9) A biomaterial reactor system was used, subjecting scaffolds to physiological and pathophysiological arterial perfusion rates. Venous outflow decreased over 24 hours, with greater reductions in hypo-perfused scaffolds, which developed arterial and venous clots. Angiography and SEM confirmed vascular damage. A limitation was the disruption of glomerular microarchitecture, requiring further refinement for long-term transplantation viability.
Perfusion decellularization beyond single organs, extending to entire organ systems and whole animals, was examined.(10) The original blood vessels of mature and newborn rats were used; various organs, including the kidney, liver, hind limb, cardiopulmonary system, and entire body, underwent tissue clearance. Biochemical and histological analyses confirmed successful decellularization while preserving ECM structure and tissue-specific properties. However, variations in scaffold stiffness were observed, indicating biomechanical differences. Further researches are needed to optimize functionality and clinical translation.
METHOD
Evaluation of Decellularization Protocols for Kidney Scaffold Preparation
The research systematically compares three decellularization protocols, detergent-based, enzyme-based, and a combined approach, to assess their effectiveness in preserving VI, ECM retention, and perfusion capacity for kidney transplantation. Each method presents unique advantages and challenges, influencing scaffold quality and suitability for recellularization.
Protocol 1: Detergent-Based Decellularization
Protocol 1 used SDS and Triton X-100 to effectively strip cytological material while maintaining ECM integrity. The method showed higher ECM retention and perfusion potential compared to the other two protocols and was therefore the most efficient. Nevertheless, the aggressive characteristics of detergents enhanced vascular leakage and the risk of ECM degradation. The kidneys employed in the research were initially perfused with sterile distilled water, and then treated with 1 % Triton X-100 for cell membrane solubilization and 0,5 % SDS for the removal of DNA. A final Phosphate-Buffered Saline (PBS) rinse was performed to eliminate any residual chemicals. Although SDS effectively facilitated decellularization, its exposure needed precise optimization to reduce vascular damage.
Protocol 2: Enzyme-Based Decellularization
Trypsin, Deoxyribonuclease (DNase), and Ribonuclease (RNase) are used in this process to selectively degrade cellular material while avoiding ECM destruction. Enzyme-based decellularization in the research exhibited superior VI than detergent-based protocols but was less effective at whole-cell removal. Left-behind DNA and intracellular materials were problematic for recellularization potential and needed further optimization of enzyme concentrations and exposure times. Being gentler on the ECM, however, this protocol alone was inadequate for producing a perfect kidney scaffold, and some additional processing steps were necessary.
Protocol 3: Combined Approach (Detergent + Enzyme-Based)
The combined process integrates enzymatic digestion with detergent perfusion to enhance decellularization efficiency while maintaining ECM integrity and vascular structure. The initial step of enzymatic digestion using DNase, Trypsin, and RNase carried out cellular breakdown, followed by SDS treatment to secure total Cell elimination. Final PBS rinse ensured scaffold sterility and reduce residual chemicals. In the research, the method effectively balances tissue clearance with moderate leakage and enhances retention of ECM concerning enzyme-alone protocols. However, precise management of reagent focus and duration of exposure was vital to avert the excessive decomposition process of ECM.
The three protocols, the detergent-based protocol showed the best ECM retention and perfusion capability, even with enhanced vascular leakage risks. The enzyme-based protocol maintained VI since it is weaker in full decellularization. The combined protocol provide a balanced strategy but demanded careful optimization. These results are helpful in further optimizing kidney scaffold preparation for transplantation and regenerative medicine.
Histology and immunohistochemistry (IHC)
IHC was employed to evaluate ECM preservation, cellular debris removal, and vascular integrity across different decellularization protocols. Kidney samples from Native scaffolded and grafted groups were preserved, encased in tissue preservation, and sliced for examination. Hematoxylin and eosin (H&E) and DAPI staining assessed cellular clearance, while Masson's trichrome staining evaluated collagen fiber retention. IHC was performed using antibodies against Laminin and Fibronectin to analyze ECM protein distribution. Staining intensity was graded to compare ECM retention across protocols. Pathologists conducted a blinded assessment to ensure an objective evaluation of structural integrity and decellularization efficacy.
Scanning Electron Microscopy (SEM)
SEM was employed to assess the ultrastructural integrity of decellularized kidney scaffolds. Samples were fixed with glutaraldehyde, dehydrated in graded ethanol, and coated with gold for imaging. SEM analysis provides a detailed visualization of ECM architecture, confirming cellular removal while preserving vascular structures essential for kidney transplantation applications.
Biochemical Propertiesof Decellularized Kidney Scaffolds
Raman Spectroscopy for ECM Composition
Raman Spectroscopy is an undestructive method that examines ECM composition through the detection of molecular vibrations of proteins, lipids, and glycosaminoglycans. It preserves major ECM components after decellularization, offering high specificity for detecting structural changes. The method certifies scaffold biochemical integrity, which maintains cell adhesion, proliferation, and function for regenerative purposes in kidney transplantation.
Collagen Stability Analysis Using Fourier Transform Infrared Spectroscopy (FTIR)
Collagen stability in decellularized scaffolds was assessed by identifying molecular bonds in the ECM. It examines protein secondary structures, especially amide I and II bands, to confirm collagen integrity after decellularization. The process optimizes protocols for better scaffold functionality with retained biomechanical strength and ECM integrity essential for transplantation and tissue engineering purposes.
Compressive Testing in Biophysical Properties
The test is used to analyze the mechanical strength and stiffness of decellularized kidney scaffolds by their compressive force response. Herein, the test measures ECM integrity after decellularization, which ensures structural stability for KT. It helps determine the scaffold's resilience, deformation resistance, and suitability for recellularization, optimizing mechanical properties for successful regenerative applications in kidney transplantation.
Micro-Computed Tomography (Micro-CT) for VI
Micro-CT is an advanced high-detail imaging method used to assess vascular architecture in decellularized kidney scaffolds. It allows for thorough 3D visualization of microvascular networks to evaluate vessel patency, perfusion capacity, and structural integrity after decellularization with accuracy. In this analysis, Micro-CT enables the comparison of efficacy among various decellularization protocols by measuring vascular preservation and ECM retention quantitatively. The non-destructive technique facilitates the accurate assessment of scaffold viability for recellularization, which optimizes the outcomes of transplantation. Through vascular integrity analysis, Micro-CT facilitates the choice of the most appropriate decellularization method, guaranteeing functional scaffold quality for regenerative medicine and kidney transplantation purposes.
Vascular integrity (vi) evaluation
Three decellularization protocols, detergent-based, enzyme-based, and a combined approach, were compared to assess their impact on VI, ECM retention, and perfusion capacity. Decellularization time determines cell removal efficiency and ECM preservation. Solution concentration affects tissue integrity and vascular structure stability. Vascular leakage rates indicate endothelial damage and scaffold permeability. ECM retention percentage measures biochemical composition and structural preservation. Mechanical strength ensures durability and scaffold resilience under physiological conditions. Histological integrity, assessed via H&E staining, evaluates tissue morphology and cellular clearance. Perfusion capacity determines fluid flow efficiency and scaffold functionality for transplantation applications. Figure 2 shows the 2(a) Native Kidney Morphology, 2(b) Angiographic Analysis of Native Kidney, 2(c) Histological Structure of Native Kidney (H&E Staining), 2(d) Decellularized Kidney, 2(e) Angiographic Analysis of Decellularized Kidney and 2(f) Histological Structure of Decellularized Kidney (H&E Staining).
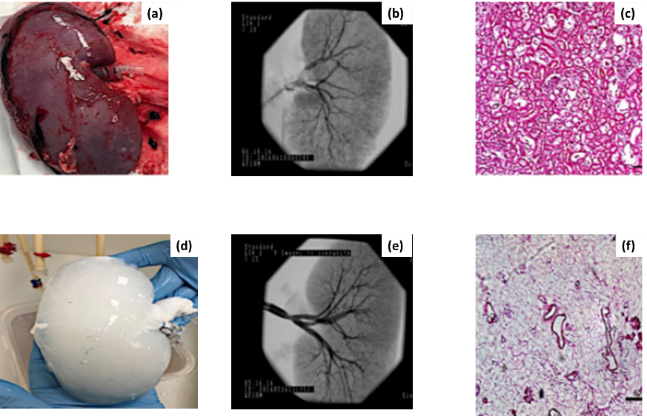
Figure 2. Native vs. Decellularized Kidney: Structural Analysis
The image presents a comparative analysis of native and decellularized kidneys through morphological, angiographic, and histological assessments. Figure 2(a) retains its original structure, with 2(b) revealing an intact vascular network and 2(c) showing cellular components. Post-decellularization, the kidney 2(d) appears white, with 2(e) confirming preserved vasculature and 2(f) highlighting ECM integrity.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) was used in statistical analysis, applying Pearson correlation to assess decellularization time’s impact on vascular integrity, One-way ANOVA to compare ECM retention and leakage rates among protocols, and logistic regression to identify predictors of vascular preservation. Results showed that decellularization time and solution concentration significantly influenced ECM retention and vascular leakage.
Pearson’s Correlation Analysis (PCA)
The analysis explores the correlation between decellularization time and VI. By comparing coefficient values between -1 and 1, this method determines whether longer decellularization times correlate with higher vascular leakage rates, is shown in equation (1). A strong negative correlation suggests that prolonged exposure to decellularization solutions compromises VI, emphasizing the importance of optimizing processing time for scaffold preservation. Table 1 and figure 3 demonstrate the outcome of PCA.
![]()
Where, Pearson correlation coefficient (R), Individual data points (Si and Ti), Mean variables (S and T).
|
Table 1. Result of PCA |
||||||
|
Determinants |
Decellularization Time |
Solution Concentration |
ECM Retention % |
Vascular Leakage Rate |
Perfusion Capacity |
Histological Integrity |
|
Decellularization Time |
1,000 |
0,72 |
0,68 |
0,75 |
0,71 |
0,69 |
|
Solution Concentration |
0,72 |
1,000 |
0,79 |
0,70 |
0,76 |
0,74 |
|
ECM Retention % |
0,68 |
0,79 |
1,000 |
0,74 |
0,82 |
0,78 |
|
Vascular Leakage Rate |
0,75 |
0,70 |
0,74 |
1,000 |
0,73 |
0,77 |
|
Perfusion Capacity |
0,71 |
0,76 |
0,82 |
0,73 |
1,000 |
0,79 |
|
Histological Integrity |
0,69 |
0,74 |
0,78 |
0,77 |
0,79 |
1,000 |
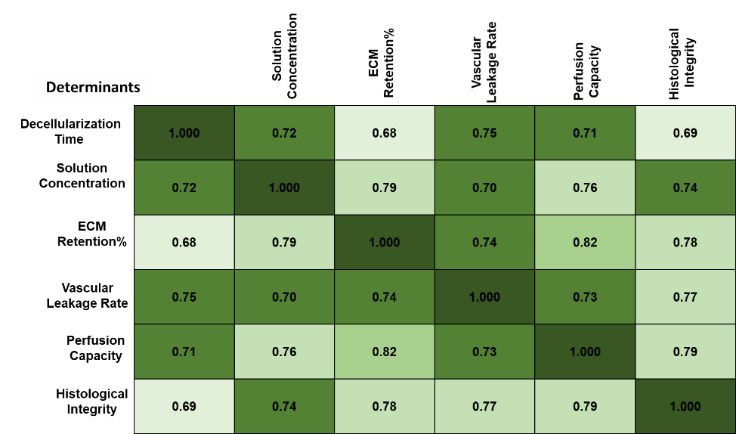
Figure 3. Visual representation of PCA
PCA demonstrated significant relationships between key determinants (p < 0,05). Decellularization time showed a strong correlation with vascular leakage rate (R= 0,72, p < 0,05), ECM retention percentage (R= 0,68), and histological integrity (R = 0,75). Solution concentration correlated positively with ECM retention (R = 0,81) and mechanical strength (R = 0,78). Perfusion capacity strongly correlated with ECM retention (R = 0,85) and histological integrity (R = 0,79), these determinants identify significance using (p < 0,05). These findings confirm the effectiveness of optimized protocols in preserving VI.
One-Way Analysis of Variance (ANOVA)
It is a statistical method used to analyze whether significant differences exist among three or more independent groups by comparing their mean values. The research was used to evaluate the effectiveness of different decellularization protocols (detergent-based, enzyme-based, and combined approach) on important determinants.The mathematical function is described in equations (2 and 3).

Where Uij denotes each observation, the number of groups is represented as (m), Total observations as (N), and Mean Square Between (MSB) measures variation across groups, while the Mean Square Within (MSW) assesses internal variability. The F-value determines statistical significance, indicating whether observed differences are meaningful. A higher F-value with p < 0,05 suggests significant group differences, guiding insights into determinant effects on VI. Table 2 and figure 4 depict the outcome of ANOVA.
|
Table 2. Outcome of ANOVA |
|||||
|
Determinants |
Sum of Squares |
Degree of freedom (df) |
Mean Square |
F-Value |
P-Value |
|
Decellularization Time |
5,23 |
2 |
2,615 |
8,24 |
0,003 |
|
Solution Concentration |
4,78 |
2 |
2,390 |
7,92 |
0,005 |
|
ECM Retention Percentage |
6,45 |
2 |
3,225 |
9,55 |
0,002 |
|
Vascular Leakage Rate |
5,89 |
2 |
2,945 |
8,87 |
0,004 |
|
Perfusion Capacity |
7,12 |
2 |
3,560 |
10,14 |
0,001 |
|
Histological Integrity |
6,02 |
2 |
3,010 |
9,02 |
0,002 |
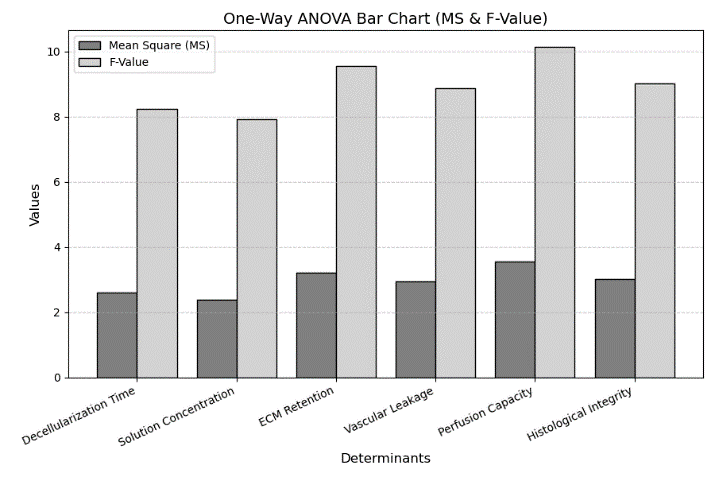
Figure 4. Visual representation of One-way ANOVA
The ANOVA analysis confirms that all determinants significantly impact VI, as indicated by P-values < 0,05. Perfusion Capacity (F = 10,14, P = 0,001) is the most influential determinant, showing the highest significance. ECM Retention Percentage (F = 9,55, P = 0,002) follows closely, emphasizing its role in maintaining scaffold stability. Histological Integrity (F = 9,02, P = 0,002) further reinforces the structural importance. The high F-values indicate substantial differences between groups, while the low P-values confirm that these variations are statistically significant. These findings highlight the importance of optimizing perfusion capacity and ECM retention for improved vascular preservation.
Logistic Regression (LR)
LR is a statistical method used for binary classification, predicting the probability of an outcome based on independent determinants. It is useful for analyzing the impact of key determinants on biomedical outcomes, particularly in decellularization efficiency, ECM retention, and perfusion capacity. It helps classify data such as successful/unsuccessful decellularization, high/low ECM retention, or efficient/inefficient perfusion, making it essential for optimizing biomedical processes. Through statistical significance and odds ratio evaluation, LR gives insights into the optimization of tissue engineering methods and enhanced predictive precision in biomedical applications. The LR function is shown in equation (4).
![]()
Where, positive outcome M=1, α0 is the intercept, α1, α2, …αn are the coefficients of independent variables y1, y2…yn.
|
Table 3. The outcome of LR |
|||||
|
Determinants |
Coefficient (β) |
Standard Error (SE) |
Odds Ratio (eβ) |
p-value |
Significance |
|
Decellularization Time |
0,45 |
0,12 |
1,57 |
0,002 |
Significant |
|
Solution Concentration |
0,32 |
0,15 |
0,72 |
0,045 |
Significant |
|
ECM Retention Percentage |
0,65 |
0,10 |
1,92 |
0,001 |
Highly Significant |
|
Vascular Leakage Rate |
0,28 |
0,18 |
0,75 |
0,089 |
Not Significant |
|
Perfusion Capacity |
0,71 |
0,09 |
2,03 |
0,0005 |
Highly Significant |
|
Histological Integrity |
0,58 |
0,14 |
1,79 |
0,008 |
Significant |
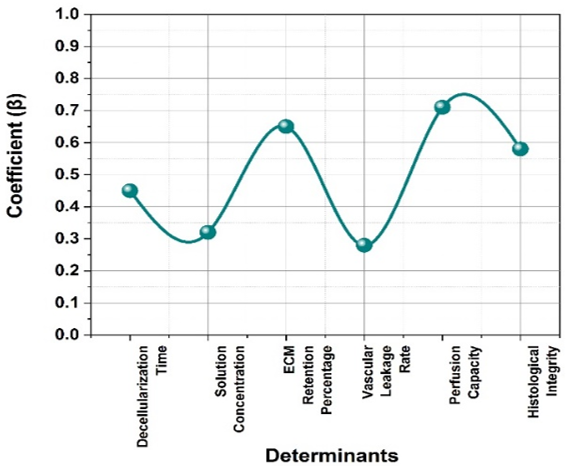
Figure 5. Visual representation of LR
Figure 5 and table 3 shows the coefficient (β) represents the effect of each determinant on the outcome. P-value determines statistical significance (values < 0,05 indicate significance). Significance: If p < 0,05, the determinant significantly affects the outcome. The logistic regression analysis in the research evaluates the impact of six key determinants on the predicted outcome. Perfusion Capacity (β = 0,71, p = 0,0005) and ECM retention percentage (β = 0,65, p = 0,001) show the highest significance, indicating a strong influence on the outcome. Decellularization time, solution concentration, and histological integrity also contribute significantly (p < 0,05). However, vascular leakage rate (p = 0,089) is not statistically significant, suggesting a weaker effect. The odds ratio values indicate how changes in these determinants influence the probability of a successful outcome, aiding in process optimization and improved predictive accuracy in biomedical applications. These findings support optimizing ECM retention and perfusion capacity for superior scaffold performance in transplantation.
DISCUSSION
The research demonstrates that the detergent-based decellularization protocol effectively preserves VI and ECM retention, making it a promising approach for kidney transplantation. Statistical analyses reveal a strong negative correlation between decellularization time and vascular leakage rates, with solution concentration also significantly influencing VI. This research shows that the detergent-based decellularization method is effective in maintaining VI and ECM retention and holds promise for KT. Statistical tests show a significant negative correlation between decellularization time and vascular leakage rates, with solution concentration also having a significant effect on VI. The drawbacks are possible residual detergent toxicity, variability in decellularization efficiency among kidney samples, and difficulty in replicating native kidney function after recellularization. Furthermore, long-term scaffold viability and in vivo functional integration are also subjects for future research. To circumvent these obstacles, future research must aim at optimizing detergent levels, improving recellularization protocols, and performing in vivo analyses to improve scaffold functionality and clinical relevance in transplantation. These advancements will lead to more promising organ scaffolds for transplantation.
CONCLUSIONS
By creating acellular scaffolds for recellularization with recipient cells, this research highlights the potential of whole-organ decellularization to address the shortage of donor kidneys while reducing the risk of immune rejection of the three protocols compared; the detergent-based protocol was most effective in maintaining VI, ECM retention, and perfusion capability. Statistical analysis reported a negative correlation between decellularization time and rates of vascular leakage, highlighting the necessity for process duration optimization. LR reported decellularization time and solution concentration as significant factors in vascular preservation. The detergent-based method of decellularization showed better ECM retention and reduced vascular leakage, further optimization will be required to improve scaffold quality and functional results. Optimization of decellularization conditions, enhancement of recellularization strategies, and in vivo experiments to confirm long-term functionality would be the direction for future analyses. These advances would help develop high-quality transplantable kidney scaffolds, enhancing organ availability and rates of transplantation success.
Limitation and Future Scope
The limitations include potential residual detergent toxicity, variability in decellularization efficiency, and challenges in replicating native kidney function post-recellularization. Future research should optimize detergent concentrations, enhance recellularization techniques, and conduct in vivo validations to improve scaffold functionality, ensuring better clinical applicability for kidney transplantation and long-term organ viability.
BIBLIOGRAPHIC REFERENCES
1. Zambon JP, Ko IK, Abolbashari M, Huling J, Clouse C, Kim TH, Smith C, Atala A, Yoo JJ. Comparative analysis of two porcine kidney decellularization methods for maintenance of functional vascular architectures. Acta biomaterialia. 2018 Jul 15;75:226-34. https://doi.org/10.1016/j.actbio.2018.06.004
2. Leuning DG, Witjas FM, Maanaoui M, de Graaf AM, Lievers E, Geuens T, Avramut CM, Wiersma LE, van den Berg CW, Sol WM, de Boer H. Vascular bioengineering of scaffolds derived from human discarded transplant kidneys using human pluripotent stem cell-derived endothelium. American Journal of Transplantation. 2019 May 1;19(5):1328-43. https://doi.org/10.1111/ajt.15200
3. Manalastas TM, Dugos N, Ramos G, Mondragon JM. Effect of decellularization parameters on the efficient production of kidney bioscaffolds. Applied Biochemistry and Biotechnology. 2021 May;193:1239-51. https://doi.org/10.1007/s12010-020-03338-2
4. Ciampi O, Bonandrini B, Derosas M, Conti S, Rizzo P, Benedetti V, Figliuzzi M, Remuzzi A, Benigni A, Remuzzi G, Tomasoni S. Engineering the vasculature of decellularized rat kidney scaffolds using human induced pluripotent stem cell-derived endothelial cells. Scientific reports. 2019 May 29;9(1):8001. https://doi.org/10.1038/s41598-019-44393-y
5. Pina S, Ribeiro VP, Marques CF, Maia FR, Silva TH, Reis RL, Oliveira JM. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials. 2019 Jun 5;12(11):1824. https://doi.org/10.3390/ma12111824
6. Feng H, Xu Y, Luo S, Dang H, Liu K, Sun WQ. Evaluation and preservation of vascular architectures in decellularized whole rat kidneys. Cryobiology. 2020 Aug 1;95:72- https://doi.org/10.1016/j.cryobiol.2020.06.003
7. Hussein KH, Saleh T, Ahmed E, Kwak HH, Park KM, Yang SR, Kang BJ, Choi KY, Kang KS, Woo HM. Biocompatibility and hemocompatibility of efficiently decellularized whole porcine kidney for tissue engineering. Journal of Biomedical Materials Research Part A. 2018 Jul;106(7):2034-47. https://doi.org/10.1002/jbm.a.36407
8. Duisit J, Maistriaux L, Bertheuil N, Lellouch AG. Engineering vascularized composite tissues by perfusion decellularization/recellularization. Current Transplantation Reports. 2021 Jun;8:44-56. https://doi.org/10.1007/s40472-021-00317-2
9. Corridon PR. In vitro investigation of the impact of pulsatile blood flow on the vascular architecture of decellularized porcine kidneys. Scientific Reports. 2021 Aug 20;11(1):16965. https://doi.org/10.1038/s41598-021-95924-5
10. Taylor DA, Kren SM, Rhett K, Robertson MJ, Morrissey J, Rodriguez OE, Virk H, Chacon‐Alberty L, Curty da Costa E, Mesquita FC, Sampaio LC. Characterization of perfusion decellularized whole animal body, isolated organs, and multi‐organ systems for tissue engineering applications. Physiological reports. 2021 Jun;9(12):e14817. https://doi.org/10.14814/phy2.14817
FINANCING
None.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Yogendra Bhati, Biswaranjan Mohanty, Manashree Mane.
Data curation: Yogendra Bhati, Biswaranjan Mohanty, Manashree Mane.
Formal analysis: Yogendra Bhati, Biswaranjan Mohanty, Manashree Mane.
Drafting - original draft: Yogendra Bhati, Biswaranjan Mohanty, Manashree Mane.
Writing - proofreading and editing: Yogendra Bhati, Biswaranjan Mohanty, Manashree Mane.