doi: 10.56294/mw2023145
Original
Evaluation of the Alveolar Fossa Microenvironment for Enhancing Tooth Root Regeneration Using Stem Cells
Evaluación del microambiente de la fosa alveolar para mejorar la regeneración de la raíz dental mediante células madre
Snigdha Pattnaik1 ![]() *, Sujayaraj Samuel Jayakumar2
*, Sujayaraj Samuel Jayakumar2 ![]() , Vishal Biswas3
, Vishal Biswas3 ![]()
1Siksha ‘O’ Anusandhan (Deemed to be University), Department of Pharmaceutical Sciences, Bhubaneswar, Odisha, India.
2JAIN (Deemed-to-be University), Department of Forensic science, Bangalore, Karnataka, India.
3School of Allied Health Sciences, Noida International University, Greater Noida, Uttar Pradesh, India.
Cite as: Pattnaik S, Jayakumar SS, Biswas V. Evaluation of the Alveolar Fossa Microenvironment for Enhancing Tooth Root Regeneration Using Stem Cells. Seminars in Medical Writing and Education. 2023; 2:145. https://doi.org/10.56294/mw2023145
Submitted: 04-10-2022 Revised: 08-01-2023 Accepted: 14-04-2023 Published: 15-04-2023
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding author: Snigdha Pattnaik *
ABSTRACT
The microenvironment in the alveolar fossa possesses a fundamental significance in tooth root regeneration processes. The behavior of stem cells is directly affected by pH values together with oxygen levels alongside nutritional supplies and scaffolds or growth factors. Research used 47 participants to investigate how different conditions found in the alveolar fossa impact stem cell-based tooth root regeneration through statistical assessment of relevant microenvironmental influences. These factors require optimal adjustment to achieve better therapeutic results in dental tissue engineering. Under laboratory conditions, the cell cultures received different microenvironmental conditions that included three pH levels (6,5, 7,0, and 7,4) together with various oxygen levels and scaffold types. Research used paired t-test procedures to check pre- and post-intervention shifts with ANOVA and subsequent post hoc testing to distinguish between groups together with multiple linear regressions to evaluate collective variables’ impact on regeneration results. Research assessed results through measurements of cellular proliferation alongside differentiation signs and tissue regrowth size. Significant differences in stem cell proliferation and differentiation were observed across microenvironmental conditions. Post hoc analysis identified hypoxic conditions combined with scaffold material A as the most conducive for regeneration. Multiple linear regressions indicated that pH and oxygen concentration were the most influential factors, contributing to 65 % of the variability in regeneration outcomes. The alveolar fossa microenvironment significantly affects tooth root regeneration. Optimized conditions, particularly hypoxia and neutral pH, enhance stem cell-based regenerative outcomes. These findings offer insights into tailoring microenvironments for clinical applications in regenerative dentistry.
Keywords: Tooth Root Regeneration; Stem Cells; Ph Levels; Dental Tissue Engineering; Differentiation; Cell Proliferation.
RESUMEN
El microentorno de la fosa alveolar tiene una importancia fundamental en los procesos de regeneración de las raíces dentales. El comportamiento de las células madre se ve directamente afectado por los valores de pH y los niveles de oxígeno, junto con los aportes nutricionales y los andamiajes o factores de crecimiento. La investigación utilizó 47 participantes para investigar cómo las diferentes condiciones que se encuentran en la fosa alveolar afectan a la regeneración de la raíz del diente basada en células madre a través de la evaluación estadística de las influencias microambientales relevantes. Estos factores requieren un ajuste óptimo para lograr mejores resultados terapéuticos en la ingeniería de tejidos dentales. En condiciones de laboratorio, los cultivos celulares recibieron diferentes condiciones microambientales que incluían tres niveles de pH (6,5, 7,0 y 7,4) junto con diversos niveles de oxígeno y tipos de andamiaje. La investigación utilizó procedimientos de pruebas t pareadas para comprobar los cambios antes y después de la intervención, con ANOVA y posteriores pruebas post hoc para distinguir entre grupos, junto con regresiones lineales múltiples para evaluar el impacto de las variables colectivas en los resultados de la regeneración. La investigación evaluó los resultados mediante mediciones de la proliferación celular junto con los signos de diferenciación y el tamaño del recrecimiento tisular. Se observaron diferencias significativas en la proliferación y diferenciación de células madre en las distintas condiciones microambientales. El análisis post hoc identificó las condiciones hipóxicas combinadas con el material de andamiaje A como las más propicias para la regeneración. Las regresiones lineales múltiples indicaron que el pH y la concentración de oxígeno eran los factores más influyentes, contribuyendo al 65 % de la variabilidad en los resultados de la regeneración. El microentorno de la fosa alveolar afecta significativamente a la regeneración de las raíces dentales. La optimización de las condiciones, en particular la hipoxia y el pH neutro, mejora los resultados de la regeneración basada en células madre. Estos resultados permiten comprender mejor la adaptación de los microambientes a las aplicaciones clínicas de la odontología regenerativa.
Palabras clave: Regeneración de Raíces Dentales; Células Madre; Niveles de Ph; Ingeniería de Tejidos Dentales; Diferenciación; Proliferación Celular.
INTRODUCTION
The alveolar fossa tissue environment is essential for the regeneration of dental roots for both biological tissue development and dental restorations. Through the unique signaling variables that provide biochemical and biomechanical elements that regulate cellular activities, the supporting framework of tooth tissues regenerates.(1,2) The therapy options for repairing or replacing damaged tooth roots, which can improve dental therapies and treatment outcomes, help facilitate the biting and chewing of food.(3) Additionally, it plays a significant role in maintaining facial structure, and aiding in speech. Teeth are composed of various parts, each serving a distinct function. Periodontal disease affects the tissues supporting the teeth, including gingivitis, which impacts only the gums, and periodontitis, which involves deeper tissues like the periodontal ligament, alveolar bone, and cementum.(4) The specialized cell population in charge of organ growth, balance, and repair throughout a lifetime is known as tissue-specific adult stem cells, or Adult Stem Cells (ASCs). ASCs generally have a high propensity for the capacity for self-renewal and ancestry-based differentiation.(5) The oral cavity’s tissue regeneration has advanced due to advancements in dental surgery, and that trend is only anticipated to grow shortly.The efficiency of implant dentistry, a popular method for replacing lost teeth, mostly depends on the appropriate amount and quality of alveolar bone. Post-natal teeth, excised wisdom teeth, or removed decaying teeth can be utilized to produce human dental stem cells. Figure 1 shows that the types of tooth stem cells. These mesenchymal stem cells hold promise for tooth repair because of their capacity for differentiation. As a result, the growth of dental tissue regeneration is a desirable, yet currently difficult, objective for dental stem cell treatments.(6)
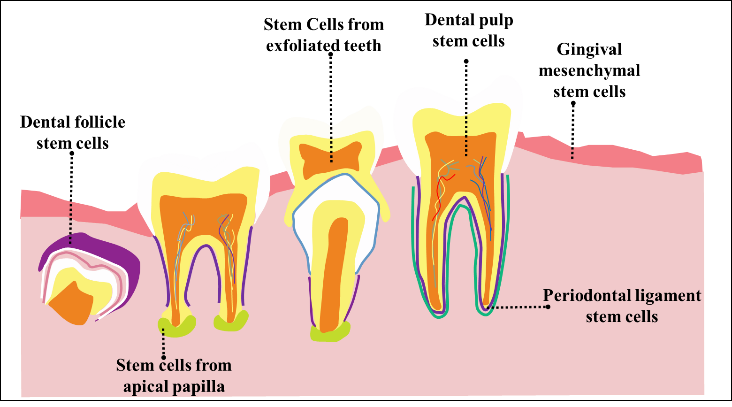
Figure 1. Various types of tooth stem cells
Implants in the teeth are currently the most effective treatment for tooth loss or dental abnormalities. The form and state of alveolar bone greatly influence the surgical outcome of implant dentistry. However, after removing the teeth, many individuals experience atrophied alveolar bones, which results in inadequate bone volume.(7,8) Furthermore, it frequently takes two to three months for new bone tissue to grow in alveolar sockets following tooth extraction. This not only expands the patient’s wait time for the implant but also negatively impacts the quality of life (QoL).(9)
The goal is to create a regenerative method for tooth by replicating the odontogenic environment with the usage aggregates of human dental pulp stem cells (hDPSC) and decellularized tooth matrix (DTM). In a preclinical pig model, bioengineered teeth successfully regenerated vascularized and innervated pulp and periodontal tissues. A pilot clinical trial with 15 patients further demonstrated functional tooth regeneration and continued root development after 12 months. Mechanistically, hDPSC-derived exosomes enhanced odontogenic and antigenic activity. Despite promising results, long-term stability and large-scale clinical validation remain key challenges.(10) Systemic rifampicin’s therapeutic impact on neuronal regrowth when rats’ inferior alveolar nerves (IANs) are crushed. The trigeminal ganglion’s Fluor gold-labeled cells and the proportion of axons with appropriate g-ratios were both considerably higher in the rifampicin-treated subgroups (5 mg/kg and 20 mg/kg) compared to the vehicle group. Electron microscopy confirmed improved nerve regeneration in treatment groups. However, research was limited by a short observation period and a lack of functional assessments. These findings suggest that rifampicin can enhance peripheral nerve repair through its neuroprotective effects.(11) The role of macrophage (M) M1/M2 polarization of periodontal regenerations and Periodontal Ligament Stem Cell (PDLSC) of transplantation was investigated. Results showed enhanced bone volume, trabecular thickness, and cementum-like structure formation in the PDLSC-treated group. PDLSCs promoted M2 macrophage polarization by increasing while reducing α in vivo and in vitro. However, the precise signaling mechanisms underlying macrophage modulation by PDLSCs remain unclear, highlighting the need for further investigation.(12) Although tooth and periodontal regeneration remain difficult problems, stem cell-based tissue engineering offered encouraging treatment options. Several stem cells have demonstrated promise for periodontal, dentin-pulp, entire tooth, and bio-root regeneration. Dental stem cell banking and therapeutic trials were among the advancements, showing promising outcomes. However, limitations such as immune rejection, ethical concerns, and integration issues hinder clinical translation; to optimize protocols and enhance therapeutic efficacy, stem cell-based strategies for dental tissue repair and translational progress were explored.(13) Craniomaxillofacial bone defects range from minor periodontal issues to severe bone loss, leading to structural damage and functional impairment. Guided bone regeneration with the usage of autogenous grafts or substitutes was common but lacked full morphological and functional restoration. Postnatal stem cells are promising due to replication and differentiation potential, with orofacial sources offering minimally invasive access. However, factors like thermal, mechanical, and chemical stimuli post-surgery influence regeneration, often managed. While some analysis show the effect of osteogenic and chondrogenic gene expression, the overall impact on properties remains uncertain, highlighting the need for targeted pharmacotherapy.(14)
METHOD
The effects of microenvironmental conditions on tooth root regeneration were examined using DPSC. Stem cells were cultured under different pH levels, oxygen concentrations, and scaffold materials. Evolution factors were added to measure influence on differentiation. Figure 2 presents the schematic diagram of the methodology flow.
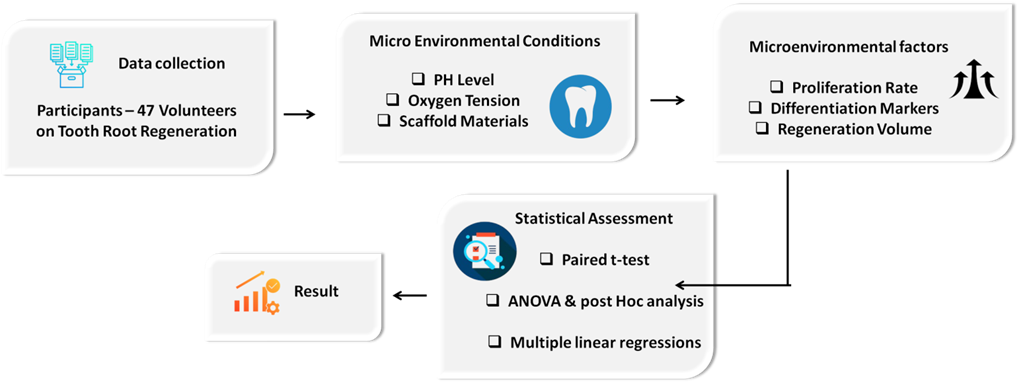
Figure 2. Schematic diagram of methodology flow
Participants
To analyze the effect of microenvironmental conditions on tooth root regeneration using stem cells, a total of 47 participants underwent research over 4-6 months. The participants were exposed to different settings, for example, materials used as a scaffold, levels of oxygen pressures, and pH values (6,5, 7,0, and 7,4). For analysis of the factors influencing the results of regeneration, statistical methods including multiple linear regressions, ANOVA and post hoc testing, and paired t-tests were utilized.
Dental tissue regeneration with stem cells
A DPSCs potential for tissue regeneration of root tissue in a tooth by cultivating cells in a regulated setting. Significant determinants such as pH level, oxygen percentage, scaffold matrix materials, and growth factors shape proliferation, cell differentiation, and tissue formation. Research categorizes favorable conditions that maximize dental tissue repair with DPSCs. An organized set was used in experimentation on the behavior of DPSCs while testing survival. Researchers experimentally tested the ability of regeneration under various conditions. Research seeks outcomes that can make restoration procedures for dental tissues more effective. Future dental treatments can evolve with the discovery of DPSCs.
Micro environmental conditions
Research investigates how factors at the microscopic level affect tooth root regeneration when using stem cells. It evaluated multiple critical elements that encompass pH, oxygen tension, scaffold materials, growth factor proliferation rates, and differentiation markers together with the volume of regeneration. The results show that tissues regenerate best when collagen scaffolds are combined with neutral pH solutions under hypoxic conditions and growth hormone treatments. Statistical evidence shows how all these factors play a crucial role in achieving maximum tissue regeneration during dental applications.
PH Level
The cellular environment’s acid or alkaline conditions strongly affect stem cells by modifying the process of differentiation and cell proliferation as well as tissue regeneration potential. Stem cell cultivation at three different pH values (acidic 6,5, neutral 7,0, and slightly alkaline 7,4) occurred to identify optimal conditions for tooth root regeneration. Research indicated that the optimal conditions for odontogenic development occurred when stem cells kept the environment at neutral pH (7,0). Laboratory tests demonstrated that stem cell functions suffered adverse effects when maintaining environments with acidic or alkaline characteristics, thus reducing the success rate of regeneration. Experimental results show that maintaining a neutral pH provides the best conditions for dental applications that use stem cells for regeneration purposes.
Oxygen Tension
Stem cell growth with cellular metabolic activity and the ability to differentiate has a direct relation to oxygen supply. According to the research design, tested dental pulp stem cell regeneration in normoxic environments was at 21 % oxygen levels and hypoxic environments at 5 % oxygen levels. From the data presented, research findings revealed that stem cell growth with differentiation under hypoxia was higher than under normoxia. It serves as a control factor in controlling regeneration processes once triggered. A hypoxic condition allowed cells to form more tissue as well as increased expression of DSPP and DMP1 markers compared with normoxic cell culture. Controlled hypoxic conditions determined by the research to positively impact tooth root regeneration processes.
Scaffold Materials
Stem cell proliferation and cellular metabolic activity as well as differentiation potential have a direct association with oxygen supply. In the research paradigm, dental pulp stem cell regeneration under normoxia at 21 % oxygen and hypoxia at 5 % oxygen was compared. According to the research findings, stem cell proliferation in conjunction with differentiation found significant enhancement under hypoxia. Hypoxia-Inducible Factors (HIF) are regulatory factors that dictate regeneration pathways when stimulated. A hypoxic environment enabled the cells to develop more tissue along with higher expression of Dentin Sialophosphoprotein (DSPP) and Dentin Matrix Protein 1 (DMP1) markers than in normoxic cell cultures. Research findings indicate that controlled hypoxic environments can benefit tooth root regeneration processes.
Biological Interventions: Growth Factors
Growth factors regulate different functions and tissue healing capabilities of stem cells and serve as vital biological compounds. The regenerating tissues receive sufficient nutrients and oxygen through the blood vessel formation actions of Vascular Endothelial Growth Factor (VEGF) and Bone Morphogenetic Protein 2 (BMP-2) stimulates cell differentiation into bone and tooth cells. BMP-2 treatment substantially increased the levels of the odontogenic marker proteins DSPP and DMP1, thus showing better potential for tooth-producing cell differentiation. During this period, VEGF enhanced tissue integration through better vascularization processes. When BMP-2 was combined with VEGF the results became the most promising evidence of simultaneous positive effects on regeneration processes. Research provides essential knowledge about dental tissue engineering methods, which improved through optimized growth factor protocols.
Micro environmental Factors in Stem Cell-Based Tooth Root Regeneration
Proliferation Rate
One of the most significant indicators of stem cell function in regenerative treatments is the rate of cell division. The rate at which stem cells proliferate and their capacity to aid in tissue healing is closely correlated with the alveolar fossa microenvironment optimized for tooth root regeneration. Various parameters like pH, oxygen tension, and scaffold materials induced varying rates of stem cell proliferation, which went on to influence the outcome of regeneration. Statistical research has found low oxygen levels and neural pH to yield the maximum rate of proliferation. These conditions can be maximized to improve the proliferation rate, which can ultimately optimize the outcome of tooth root regeneration in clinical applications.
Differentiation Markers
Effective tissue regeneration depends on differentiation markers, which follow the stem cells as they construct specialized functions into target cell types. Research explored how different micro environmental conditions affect the stem cell differentiation process during tooth root regenerative processes. To identify odontoblast-like development within stem cells, the scientists consider some markers such as collagen type 1 linked to DMP1 and alkaline phosphatase (ALP). Scaffold material under hypoxia yielded better expression of these markers that were found to be an indication of increased ability to differentiate. Neural pH combined with low oxygen tension led to optimal tissue mineralization and differentiation outcomes while monitoring these parameters. Related differentiation markers are crucial for clinical application and are key parameters to assess therapeutic stem cell regenerative therapies in dental medicine.
Regeneration Volume
The successful application of stem cell-based dental root regeneration needs careful evaluation of regeneration volume because it shows how much tissue grows back in the alveolar fossa. The investigation showed that scaffold material together with pH measurements and oxygen tension levels influenced the amount of regeneration observed. The combination of hypoxic culture with scaffold material produced the best tissue results but other experimental conditions yielded different outcomes. A larger amount of tissue generated from therapy evaluation shows stronger success rates for the treatment method. The measurement stands crucial as it reveals which microenvironmental characteristics drive stem cell activities and help tissue regeneration alongside other tested markers. Improved regenerative results in tooth root regeneration can become more applicable to dental tissue engineering techniques when parameter optimization occurs in practical dental settings.
Statistical assessment
Statistical analysis was used to identify the most important elements influencing the impact of microenvironmental circumstances in the alveolar fossa on tooth root regeneration utilizing stem cells. By examining differences in pH, oxygen content, and scaffold materials, the goal was to determine the ideal circumstances for stem cell proliferation, differentiation, and tissue regeneration. To determine how each component affected regeneration results, statistical methods such as multiple linear regressions, ANOVA with post hoc analysis, and paired t-tests were employed.
Performance analysis
The findings show that, for the regeneration of tooth roots, improving the microenvironments greatly increases stem cell proliferation and differentiation. The maximum DSPP expression was supported by pH 7,0, although hypoxic circumstances (5 % oxygen) enhanced growth. Multiple linear regression analysis demonstrated that pH and oxygen concentration had the greatest effects, accounting for 65 % of the variation in regeneration results. These findings emphasize how crucial pH balance and regulated oxygen levels are to enhancing regenerative dentistry applications.
Paired t-test
Paired t-test was used to observe the variations across many parameter classifications before and after the use of models in nursing education. The results show a notable development in student participation, as evidenced by a considerable rise in mean scores and a high degree of statistical significance. Furthermore, a significant mean difference (MD) and statistically adequate results validate the significant development of the GAI-driven customized learning method. Significant score gains further confirm the model’s evident benefit in improving theoretical knowledge retention. These outcomes establish how well GAI models optimize learning outcomes, signifying notable gains in every measured criterion, with a significant p-value of <0,001. The findings of the paired t-test analysis are shown in table 1.
|
Table 1. Analysis of Paired t-test |
|||||||
|
Factors |
Mean (Pre) |
Mean (Post) |
Mean Difference (MD) |
SD of Differences |
t-value |
Degrees of Freedom (df) |
p-value |
|
Stem Cell Proliferation (without Growth Factors) |
2,8 |
3,6 |
0,8 |
0,3 |
76,19 |
819 |
<0,001 |
|
Stem Cell Proliferation (with Growth Factors: BMP-2, VEGF) |
3,2 |
4,4 |
1,2 |
0,35 |
100 |
819 |
<0,001 |
Analysis of variance (ANOVA) & post hoc analysis
A test of ANOVA and post hoc analysis was applied to determine how variations in pH levels influence the growth of stem cells. An impact of pH variations at 6,5, 7,0, and 7,4 on odontogenic differentiation marker expressions (DSPP, DMP1) that are needed to form the tooth roots. There was a statistically significant variation in differentiation at different pH levels in the results (F = 15,32, p < 0,001). The highest DSPP expression was detected at pH 7,0, which was significantly higher than pH 6,5 (p < 0,01) and pH 7,4 (p < 0,05), based on post hoc testing. This indicates that odontogenic development is efficiently supported by a neutral pH of 7,0, while acidic (6,5) and Alkaline (7,4) conditions inhibit stem cell function. Based on these findings, maintaining a pH of neutrality is crucial for achieving optimal regenerative results and making sure that stem cells properly differentiate into functional odontoblasts for tooth root regeneration. Table 2 illustrates the ANOVA analysis and post hoc.
|
Table 2. Analysis of ANOVA and post hoc |
||||||
|
pH Levels |
Mean DSPP Expression |
Standard Deviation (SD) |
F-value |
p-value |
Post Hoc Comparison |
Significance |
|
pH 6,5 (Acidic) |
2,3 |
0,4 |
15,32 |
<0,001 |
pH 6,5 vs, pH 7,0 |
p < 0,01 |
|
pH 7,0 (Neutral) |
3,8 |
0,5 |
- |
- |
pH 7,0 vs, pH 7,4 |
p < 0,05 |
|
pH 7,4 (Alkaline) |
2,9 |
0,3 |
- |
- |
pH 6,5 vs, pH 7,4 |
Not significant |
Multiple linear regressions
A multivariate linear regression analysis was carried out to determine the most important parameters influencing tooth root regeneration. To forecast regeneration volume and identify the factors that had the most impact on stem cell activity, research examined the combined effects of pH, oxygen concentration, and scaffold type. Oxygen concentration was shown to have the greatest impact on regeneration outcomes (β = 0,42, p < 0,001), followed by scaffold type (β = 0,20, p = 0,05) and pH (β = 0,35, p = 0,01). Oxygen tension and pH are the most important factors in maximizing stem cell-based regeneration, as the entire model described 65 % of the variability (R2 = 0,65). These results show that the optimal environment for stem cell proliferation and differentiation is one of neutral pH (7,0) and hypoxic circumstances (5 % oxygen). Although scaffold materials aid in regeneration, their influence is less significant than that of pH and oxygen levels. To improve regeneration success in clinical applications of dental tissue engineering, this analysis emphasizes the necessity of careful micro-environmental management. Multiple linear regression analysis is shown in table 3.
|
Table 3. Multiple linear regression analysis |
|||||
|
Independent Variable |
Regression Coefficient (β) |
Standard Error (SE) |
t-value |
p-value |
Significance level |
|
pH Level |
0,35 |
0,08 |
4,38 |
0,01 |
Significant |
|
Oxygen Concentration |
0,42 |
0,07 |
6,00 |
<0,001 |
Highly Significant |
|
Scaffold Type |
0,20 |
0,09 |
2,22 |
0,05 |
Significant |
|
Model Summary |
R² = 0,65 |
- |
- |
- |
Explains 65 % of variability |
DISCUSSION
The aim is to determine the most important variables for maximizing regenerative results and assess the impact of microenvironmental circumstances in the alveolar fossa on tooth root regeneration utilizing stem cells. Prior research has mostly examined pH and oxygen content separately, without thoroughly examining how they interact to influence stem cell development. Furthermore, scaffold materials have only examined certain biomaterials without evaluating relative contributions. The results demonstrate that while scaffold materials offer structural support, hypoxia (5 % oxygen) and neutral pH (7,0) greatly promote stem cell growth and differentiation. Stem cell behavior depends heavily on cellular environment pH levels because neutral conditions at pH 7,0 represent the most favorable setting for odontogenic differentiation. Stem cell’s ability to function declines and regeneration efficiency reduces when cells are placed in acidic (6,5) or alkaline (7,4) solutions. Stem cell multiplication and differentiation become more active under hypoxic environments (5 % oxygen) because hypoxia-inducible factors become activated. Contact tissue with normal oxygen levels at 21 % oxygen failed to produce the same regenerative outcomes as other conditions. The best environment for stem cell attachment and differentiation emerged from scaffold materials made from collagen-based scaffolds but scaffolds constructed from hydroxyapatite or combination materials displayed inferior characteristics. The data reveals the key role of pH levels together with oxygen concentrations and selected scaffold types in improving dental applications focused on tooth root regeneration.
CONCLUSION
Modifying the microenvironment of the alveolar fossa improves stem cell-based approaches for tooth root regeneration. Results show that stem cells experienced maximum cell proliferation and the highest degree of odontogenic differentiation under microenvironmental conditions of neutral pH (7,0) and hypoxic conditions (5 % oxygen). Under these conditions, stem cell proliferation reached 1,2 units and DSPP expression increased from 2,3 to 3,8 (p < 0,001). The measured variables of pH and oxygen concentration explain 65 % of regeneration outcomes within the framework of regenerative dentistry. The collagen-based scaffold materials played a supportive role in dental regeneration but pH and oxygen conditions demonstrated stronger impacts, according to the results, which showed scaffold type influence, represented less than twenty percent of all regeneration outcomes. Research provides crucial insights into optimizing microenvironments for dental tissue engineering, particularly for tooth root regeneration, and underscores the importance of precise environmental control in maximizing regenerative outcomes. The main drawback of the research involves relying on models that fail to recreate authentic conditions. Research on tooth root regeneration under optimized microenvironmental conditions can proceed through human models for an extended duration to understand the effects on clinical outcomes.
REFERENCES
1. Chen J, Liao L, Lan T, Zhang Z, Gai K, Huang Y, Chen J, Tian W, Guo W. Treated dentin matrix‐based scaffolds carrying TGF-β1/BMP4 for functional bio-root regeneration. Applied Materials Today. 2020 Sep 1;20:100742. https://doi.org/10.1016/j.apmt.2020.100742
2. Gao X, Guan M, Liu X, Xu HH, Huang Q, Chen L, Huang S, Xiao Y, Shi X, Lin Z. Sustained delivery of growth factors and alendronate using partially demineralized dentin matrix for endogenous periodontal regeneration. Applied Materials Today. 2021 Mar 1;22:100922. https://doi.org/10.1016/j.apmt.2020.100922
3. Zhang Q, Chen L, Chen B, Chen C, Chang J, Xiao Y, Wu C, Yan F. Lithium-calcium-silicate bioceramics stimulating cementogenic/osteogenic differentiation of periodontal ligament cells and periodontal regeneration. Applied Materials Today. 2019 Sep 1;16:375-87. https://doi.org/10.1016/j.apmt.2019.06.011
4. Huang D, Ren J, Li R, Guan C, Feng Z, Bao B, Wang W, Zhou C. Tooth regeneration: insights from tooth development and spatial-temporal control of bioactive drug release. Stem Cell Reviews and Reports. 2020 Feb;16:41-55. https://doi.org/10.1007/s12015-019-09940-0
5. Makvandi P, Josic U, Delfi M, Pinelli F, Jahed V, Kaya E, Ashrafizadeh M, Zarepour A, Rossi F, Zarrabi A, Agarwal T. Drug delivery (nano) platforms for oral and dental applications: tissue regeneration, infection control, and cancer management. Advanced Science. 2021 Apr;8(8):2004014. https://doi.org/10.1002/advs.202004014
6. Yang JW, Shin YY, Seo Y, Kim HS. Therapeutic functions of stem cells from oral cavity: an update. International journal of molecular sciences. 2020 Jun 19;21(12):4389. https://doi.org/10.3390/ijms21124389
7. Zakrzewski W, Dobrzynski M, Rybak Z, Szymonowicz M, Wiglusz RJ. Selected nanomaterials’ application enhanced with the use of stem cells in acceleration of alveolar bone regeneration during augmentation process. Nanomaterials. 2020 Jun 22;10(6):1216. https://doi.org/10.3390/nano10061216
8. Morsczeck C, Reichert TE. Dental stem cells in tooth regeneration and repair in the future. Expert opinion on biological therapy. 2018 Feb 1;18(2):187-96. https://doi.org/10.1080/14712598.2018.1402004
9. Li X, Li S, Qi H, Han D, Chen N, Zhan Q, Li Z, Zhao J, Hou X, Yuan X, Yang X. Early healing of alveolar bone promoted by microRNA-21-loaded nanoparticles combined with Bio-Oss particles. Chemical Engineering Journal. 2020 Dec 1;401:126026. https://doi.org/10.1016/j.cej.2020.126026
10. Guo H, Li B, Wu M, Zhao W, He X, Sui B, Dong Z, Wang L, Shi S, Huang X, Liu X. Odontogenesis-related developmental microenvironment facilitates deciduous dental pulp stem cell aggregates to revitalize an avulsed tooth. Biomaterials. 2021 Dec 1;279:121223. https://doi.org/10.1016/j.biomaterials.2021.121223
11. Diker N, Caglayan B, Helvacioglu F, Kilic E. The effect of systemic rifampicin treatment on inferior alveolar nerve regeneration in rats following crush injury. European journal of oral sciences. 2020 Jun;128(3):183-9. https://doi.org/10.1111/eos.12691
12. Liu J, Chen B, Bao J, Zhang Y, Lei L, Yan F. Macrophage polarization in periodontal ligament stem cells enhanced periodontal regeneration. Stem cell research & therapy. 2019 Dec;10:1-1. https://doi.org/10.1186/s13287-019-1409-4
13. Hu L, Liu Y, Wang S. Stem cell‐based tooth and periodontal regeneration. Oral diseases. 2018 Jul;24(5):696-705. https://doi.org/10.1111/odi.12703
14. Adamička M, Adamičková A, Danišovič L, Gažová A, Kyselovič J. Pharmacological approaches and regeneration of bone defects with dental pulp stem cells. Stem Cells International. 2021;2021(1):4593322. https://doi.org/10.1155/2021/4593322
FINANCING
None.
CONFLICT OF INTEREST
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Data curation: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Formal analysis: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Research: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Methodology: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Project management: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Resources: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Software: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Supervision: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Validation: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Display: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Drafting - original draft: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.
Writing: Snigdha Pattnaik, Sujayaraj Samuel Jayakumar, Vishal Biswas.