doi: 10.56294/mw2023146
ORIGINAL
Thermo-reversible hydrogel systems for customized drug release in chronic periodontal disease treatment
Sistemas de hidrogel termorreversibles para la liberación personalizada de fármacos en el tratamiento de la enfermedad periodontal crónica
Jamuna KV1 ![]() *, Abhishek
Kumar Mishra2
*, Abhishek
Kumar Mishra2 ![]() , Shakti Bedanta Mishra3
, Shakti Bedanta Mishra3 ![]()
1JAIN (Deemed-to-be University), Department of Forensic science. Bangalore, Karnataka, India.
2Noida International University, Department of Biotechnology and Microbiology. Greater Noida, Uttar Pradesh, India.
3IMS and SUM Hospital, Siksha ‘O’ Anusandhan (deemed to be University), Department of Critical Care Medicine. Bhubaneswar, Odisha, India.
Cite as: KV J, Mishra AK, Bedanta Mishra S. Thermo-Reversible Hydrogel Systems for Customized Drug Release in Chronic Periodontal Disease Treatment. Seminars in Medical Writing and Education. 2023; 2:146. https://doi.org/10.56294/mw2023146
Submitted: 18-09-2022 Revised: 30-12-2022 Accepted: 03-03-2023 Published: 04-03-2023
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding Author: Jamuna KV *
ABSTRACT
Effective drug delivery systems are crucial for treating chronic periodontal disease, where sustained and localized drug release is essential. The aim is to investigate the potential of thermo-reversible hydrogel systems for controlled drug delivery in periodontal therapy using in vitro models. Hydrogel formulations were prepared with varying drug concentrations low (1 mg/mL), medium (5 mg/mL) and high (10 mg/mL) concentrations of DOX, an antibiotic commonly used in periodontal treatments. The hydrogels were fabricated by mixing DOX withPNIPAM polymer at a constant drug-to-polymer ratio of 1:2. These hydrogel formulations were incubated at 37°C to form the gels. IVDRPs were assessed using a dialysis membrane system, simulating the drug’s release into surrounding tissues. Additionally, HGFs and PDL cells were cultured to evaluate the biocompatibility and cytotoxicity of the hydrogels using MTT assays. The results showed that hydrogels with higher drug concentrations (high, 10 mg/mL) released the drug more rapidly within the first 24 hours, whereas medium (5 mg/mL) and low (1 mg/mL) drug concentrations exhibited a sustained release over a longer duration, up to 72 hours. Cytotoxicity testing revealed no significant toxic effects on HGFs or PDL cells at all concentrations (p > 0,05), confirming the biocompatibility of the hydrogels. These findings suggest that thermo-reversible hydrogel systems with adjustable drug concentrations can be effectively used for localized, controlled drug delivery in periodontal disease treatment.
Keywords: Thermo-Reversible Hydrogel Systems; Drug Delivery; Periodontal Therapy; Human Gingival Fibroblasts (HGFs); Drug Concentrations.
RESUMEN
Los sistemas eficaces de liberación de fármacos son cruciales para el tratamiento de la enfermedad periodontal crónica, donde la liberación sostenida y localizada de fármacos es esencial. El objetivo es investigar el potencial de los sistemas de hidrogel termo-reversibles para la liberación controlada de fármacos en la terapia periodontal utilizando modelos in vitro. Se prepararon formulaciones de hidrogel con diferentes concentraciones de fármaco: baja (1 mg/mL), media (5 mg/mL) y alta (10 mg/mL) de DOX, un antibiótico utilizado habitualmente en tratamientos periodontales. Los hidrogeles se fabricaron mezclando DOX con polímeroPNIPAM a una proporción constante de fármaco a polímero de 1:2. Estas formulaciones de hidrogeles se incubaron en el laboratorio. Estas formulaciones de hidrogel se incubaron a 37°C para formar los geles. Los IVDRP se evaluaron utilizando un sistema de membrana de diálisis, simulando la liberación del fármaco en los tejidos circundantes. Además, se cultivaron HGF y células PDL para evaluar la biocompatibilidad y citotoxicidad de los hidrogeles mediante ensayos MTT. Los resultados mostraron que los hidrogeles con mayores concentraciones de fármaco (alta, 10 mg/mL) liberaban el fármaco más rápidamente en las primeras 24 horas, mientras que las concentraciones de fármaco media (5 mg/mL) y baja (1 mg/mL) mostraban una liberación sostenida durante más tiempo, hasta 72 horas. Las pruebas de citotoxicidad no revelaron efectos tóxicos significativos sobre las células HGF o PDL en todas las concentraciones (p > 0,05), lo que confirma la biocompatibilidad de los hidrogeles. Estos resultados sugieren que los sistemas de hidrogel termo-reversibles con concentraciones de fármaco ajustables pueden utilizarse eficazmente para la administración localizada y controlada de fármacos en el tratamiento de la enfermedad periodontal.
Palabras clave: Sistemas de Hidrogel Termo-Reversibles; Administración de Fármacos; Terapia Periodontal; Fibroblastos Gingivales Humanos (Hgfs); Concentraciones de Fármacos.
INTRODUCTION
CPD is an inflammatory disease that strikes the support structures of teeth with progressive destruction of the underlying tissues, leading to comorbidities.(1) The conventional therapies for CPD mainly consist of scaling and root planning and systemic antibiotics, often challenged because of their limited ability to remain in the periodontal cavity for an extended time and their systemic side effects.(2) Such thermo-reversible hydrogel systems have, to date, been limited to providing localized, controlled, and sustained drug delivery to the periodontium.(3) Thermo-reversible hydrogels are very promising as injectable systems as they are in the liquid state at room temperature and gel up on a temperature increase to body temperature.(4) Thus it is hoped that they will extend drug presence in the periodontal pocket considerably, thus allowing the drugs to exert their therapeutic efficacy while limiting any unnecessary systemic exposure. These hydrogels can also be custom tailored for specific needs allowing controlled delivery of antimicrobial agents, anti-inflammatory drugs, and tissue regenerating biomolecules, addressing the multifactorial nature of CPD.(5) Biocompatible polymers, used with such as Pluronic, chitosan, or hyaluronic acid and thermo-reversible hydrogels can afford a suitable scaffold for controlled drug delivery, bioadhesion, and regeneration of periodontal tissue.(6) The property of responding to physiological stimuli in addition assists in the fight against CPD as an advanced drug delivery apparatus in a way that helps in reducing the frequency of medicament application besides increasing patient compliance.(7) Thermo-reversible hydrogel systems, thus, summarize the drug formulation strategies, possible loading capacities, and therapeutic advantages of this therapy over conventional treatment modalities in CPD.(8) Thus, the targeting approaches gloss over the scope of the development of effective therapy for professional management. The aim is to investigate the potential of thermo-reversible hydrogel systems for controlled drug delivery in periodontal therapy using in vitro models.
The thermo-reversible PIC hydrogel for periodontal drug delivery incorporating DOX and LXA4 was investigated.(9) The PIC hydrogel was capable of sustained drug release, which kept low bacterial count inflammation and improved gingival attachment in dogs were demonstrating its safety and efficacy as a periodontal treatment. The tunable drug delivery system developed for periodontal treatment by PLGA microspheres, within thermo-reversible PIC hydrogel, was explored.(10) Results confirmed that its drug release was controlled, structurally stable, and produced no significant inflammatory response. By modifying the composition of PLGA, drug release rates were tunable and were able to retain bio-efficacy for their potential clinical applications.
The development chitosan-based injectable hydrogel for the regeneration of dental Tissues, particularly as compared to the traditional grafting, were described.(11) CS-based hydrogels exhibit biodegradability, minimal invasiveness, and enhanced biocompatibilities were alternatives for regenerative medicine and tissue engineering. The hydrogel-based drug delivery systems, emphasizing biocompatibility, tunable properties, and controlled release mechanisms were examined.(12) It highlights recent advancements, particularly in cellulose-based hydrogels, for targeted therapies across various administration routes. The findings showcase promising clinical results, underscoring hydrogels' potential for safer, more effective drug delivery with minimal side effects.
The advancements in local drug delivery for oral infections, highlighting therapeutic challenges and innovative strategies for improved efficacy were summarized.(13) The discussed specialized drug delivery systems were advantages over systemic therapy, challenges in oral application, and emerging solutions to enhanced drug retention and prolonged release. The developments in hybrid polymeric hydrogels were combining natural and synthetic polymersfor use in biomedical applications was explored.(14) It emphasizes the different biopolymers could create hydrogelsthe physico-chemical characteristics, preparation techniques, and important biological uses in tissue engineering. The process has hydrogel cross-link, new developments in natural hydrogel and their uses in regenerative medicine were examined.(15) It highlights current limitations, challenges, and future opportunities, emphasizing the need for innovative hydrogel designs to enhanced clinical translation and support next-generation regenerative therapies.
METHOD
Hydrogel formulations were prepared by mixing DOX with PNIPAM polymer at a 1:2 drug-to-polymer ratio. Three drug concentrations (1, 5, and 10 mg/mL) were tested. The hydrogels were incubated at 37°C for gel formation. Drug release was analyzed using a dialysis membrane system. Biocompatibility was assessed by culturing HGFs and PDL cells, followed by MTT assays for cytotoxicity evaluation. Drug release profiles were recorded over 72 hours, comparing sustained release among different concentrations. Statistical analysis determined significance (p > 0,05).
Sample preparation
Hydrogel Preparation Thermo-reversible hydrogels were synthesized using PNIPAM as the base polymer and DOX as the model drug. The hydrogels were prepared at three different drug concentrations: low (1 mg/mL),medium (5 mg/mL),and high (10 mg/mL), maintaining a constant drug-to-polymer ratio of 1:2. PNIPAM was dissolved in deionized water, and DOX was added to the solution under continuous stirring to ensure homogeneous dispersion. The mixtures were incubated at 37°C to induce the sol-to-gel transition, forming thermo-reversible hydrogels. In Vitro Drug Release has Drug release kinetics been evaluated using a dialysis membrane system to simulate diffusion into surrounding tissues. Hydrogels containing DOX were placed inside dialysis tubing and submerged in phosphate-buffered saline (PBS, pH 7,4) at 37°C with constant agitation. At predetermined time intervals (0, 6, 12, 24, 48 and 72 hours), aliquots of the external solution were collected, and DOX concentration was quantified using UV-visible spectrophotometry. Biocompatibility & Cytotoxicity were HGFs and PDL cells were cultured and exposed to hydrogel formulations. Cytotoxicity was assessed using MTT assays to measure cell viability. All experiments were conducted in triplicate. Table 1 summarizes the samples and preparations.
|
Table 1. Summary of sample preparations |
|
|
Materials and strategies |
Description |
|
Hydrogel Preparation |
|
|
Polymer Used |
PNIPAM |
|
Drug Used |
DOX |
|
Drug Concentrations |
Low (1 mg/mL), Medium (5 mg/mL), High (10 mg/mL) |
|
Drug-to-Polymer Ratio |
|
|
Gel Formation Condition |
1:2 |
|
In Vitro Drug Release |
|
|
Method Used |
In Vitro Drug Release |
|
Duration |
Dialysis membrane system |
|
Sampling Intervals |
72 hours |
|
Expected Drug Release Pattern |
0, 6, 12, 24, 48, and 72 hours |
|
Biocompatibility & Cytotoxicity |
|
|
Cells Tested |
Biocompatibility & Cytotoxicity |
|
Toxicity Assay Used |
HGFs and PDL Cells |
|
Evaluation Criteria |
MTT Assay |
|
Findings |
Cell viability (%) |
Biocompatibility and Cytotoxicity
Biocompatibility refers to a material's capacity for interacting with biological processes without triggering harm. Biocompatibility of a material considers basis that no significant immune responses, inflammation, or toxicity should take place during its interaction with tissues and cells. Alternatively, Cytotoxicity measures the degree of occurrence of damage to the cells by a chemical complex. The parameter is really important in biomedical applications, as toxic materials can cause cell death, damage, or inhibition of healing. Cytotoxicity is assessed with established standard assays such as the MTT assay, which reports on cell viability based on metabolic activity. Hydrogel systems considered for periodontal treatment should therefore be biocompatible and non-Cytotoxicity so as to avoid any damage to gingival and PDL cells. In the current the thermo-reversible hydrogels have shown biocompatibility, whereby the high percentage of cell viability is maintained, thus confirming that they can be safely used for localized drug delivery.
Statistical Assessment
Using IBM SPSS 25, the statistical assessment of Thermo-Reversible Hydrogel Systems for Customized Drug Release in CPD Treatment involves analyzing MTT assay results to determine cell viability. ANOVA is used to compare mean differences across multiple substances, assessing the hydrogel’s effectiveness. The Tukey post hoc test determines whether pair wise differences are statistically significant. This guarantees systematic evaluation of the biocompatibility and efficacy to help in the optimization of hydrogel formulations for controlled drug delivery in periodontal therapy.
RESULTS
Utilizing the components in this section, assess the IVDRP of DOX, MTT Assay, ANOVA Test, and Tukey’s Post Hoc Test.
IVDRP of Thermo-Reversible Hydrogels
IVDRP of thermo-reversible hydrogels demonstrates their potential for controlled, sustained drug delivery. These systems provide temperature-sensitive release, optimizing therapeutic efficacy for CPD treatment by ensuring localized, prolonged drug action, enhancing patient compliance and treatment outcomes. The in vitro drug release showed a time-dependent release of DOX from thermo-reversible hydrogels over 72 hours. At 6 hours, drug release was 10,2 % (low), 18,5 % (medium) and 35,4 % (high). By 24 hours, the release increased to 40,5 %, 58,3 % and 85,7 %, respectively. The high-concentration hydrogel reached 98,6 % release by 72 hours, while medium and low concentrations released 92,6 % and 85,3 %, respectively. The sustained release profile suggests that 24–48 hours is optimal for controlled drug delivery, balancing effective release with prolonged therapeutic action, making medium (5 mg/mL) and low (1 mg/mL) concentrations more suitable for sustained treatment. Table 2 displays the IVDRP of DOX -loaded hydrogels over 72 Hours.
|
Table 2. IVDRP of DOX -Loaded Hydrogels Over 72 Hours |
|||
|
Sampling Interval (Hours) |
Low (1mgmL) Mean±SD (%) |
Medium (5mgmL) Mean±SD (%) |
High (10mgmL) Mean±SD (%) |
|
0 |
0,0 ± 0,0 |
0,0 ± 0,0 |
0,0 ± 0,0 |
|
6 |
10,2 ± 1,5 |
18,5 ± 2,1 |
35,4 ± 3,2 |
|
12 |
22,8 ± 2,3 |
35,6 ± 2,8 |
60,1 ± 4,0 |
|
24 |
40,5 ± 3,1 |
58,3 ± 3,5 |
85,7 ± 5,2 |
|
48 |
65,7 ± 4,2 |
79,4 ± 4,1 |
95,3 ± 3,8 |
|
72 |
85,3 ± 3,8 |
92,6 ± 4,0 |
98,6 ± 2,5 |
MTT Assay
The MTT assay evaluates the cytotoxicity and cell viability of thermo-reversible hydrogel systems for drug release in CPD treatment. It measures mitochondrial activity, indicating biocompatibility and ensuring the hydrogel’s safety and efficacy for periodontal tissue regeneration and healing. This equation (1) calculates cell viability by comparing treated and control absorbance, indicating metabolic activity and cytotoxic effects in an MTT assay.
![]()
The MTT assay results indicate high cell viability for both HGF and PDL cells across all DOX concentrations. HGFs showed 97,5 % ± 2,1 %,95,2 % ± 2,5 %, and 92,8 % ± 3,0 % viability at 1,5 and 10 mg/mL, respectively, compared to 98,6 % ± 1,8 % in the control. PDL cells exhibited 96,8 % ± 1,9 %, 94,5 % ± 2,7 % and 91,3 % ± 3,4 % viability, with a control of 98,3 % ± 2,0 %. All values indicate non-toxic effects (p> 0,05). The 24–72 hour range is optimal for sustained drug release and biocompatibility, ensuring effective periodontal therapy. Table 3 illustrates the MTT assay results for cell viability.
|
Table 3. MTT Assay Results for Cell Viability |
||||
|
Cell Type |
Low(1 mg/mL) MeanSD (%) |
Medium(5 mg/mL) MeanSD (%) |
High(10 mg/mL) Mean±SD (%) |
Control (NoDrug) Mean±SD (%) |
|
HGFs |
97,5 ± 2,1 |
95,2 ± 2,5 |
92,8 ± 3,0 |
98,6 ± 1,8 |
|
PDL Cells |
96,8 ± 1,9 |
94,5 ± 2,7 |
91,3 ± 3,4 |
98,3 ± 2,0 |
ANOVA Test
The ANOVA test analyzes the statistical significance of differences in drug release rates among various thermo-reversible hydrogel formulations. It evaluates the impact of composition and temperature on release profiles, ensuring optimized, controlled delivery for effective CPD treatment. Where MSbetween represents the variance among group means, indicating explained variance, and MSwithin represents the variance within groups, indicating unexplained variance. The F-ratio E is the comparison of these variances, determining whether the differences between group means are statistically significant in equation (2).
![]()
The ANOVA test results indicate significant effects of hydrogel variables on drug release, concentration, cell viability, gelation time, and pH. Drug release percentage showed the highest significance (F = 15,67, p< 0,001), confirming a strong correlation with time and concentration. Hydrogel concentration significantly influenced release (F = 12,32, p = 0,002). Cell viability remained stable (F = 9,65, p = 0,004), indicating biocompatibility. The ANOVA test for hydrogel variables shows significant effects. Gelation time (SS = 85,30, F = 7,89, p = 0,011) and pH (SS = 32,85, F = 5,87, p = 0,019) indicate statistical significance. The sum of Squares (SS) measures variance, DegreesofFreedom (df) define independent comparisons, MeanSquare (MS) isSS/df,F-value assesses group differences, and the p-value (<0,005) indicates statistical significance, ensuring reliable results in hypothesis testing. Table 4 shows the ANOVA test results for hydrogel system variables. Figure 1 displays the outcome of the ANOVA Test.
|
Table 4. ANOVA Test Results for Hydrogel System Variables |
|||||
|
Variables |
Sumof Squares (SS) |
Degreesof Freedom (df) |
MeanSquare (MS) |
F-value |
p-value |
|
Drug Release Percentage |
450,25 |
14 |
32,16 |
15,67 |
< 0,001 |
|
Hydrogel Concentration |
305,10 |
14 |
21,79 |
12,32 |
0,002 |
|
Cell Viability |
120,45 |
19 |
6,34 |
9,65 |
0,004 |
|
Gelation Time |
85,30 |
14 |
6,09 |
7,89 |
0,011 |
|
pH of Hydrogel Medium |
32,85 |
14 |
2,35 |
5,87 |
0,019 |
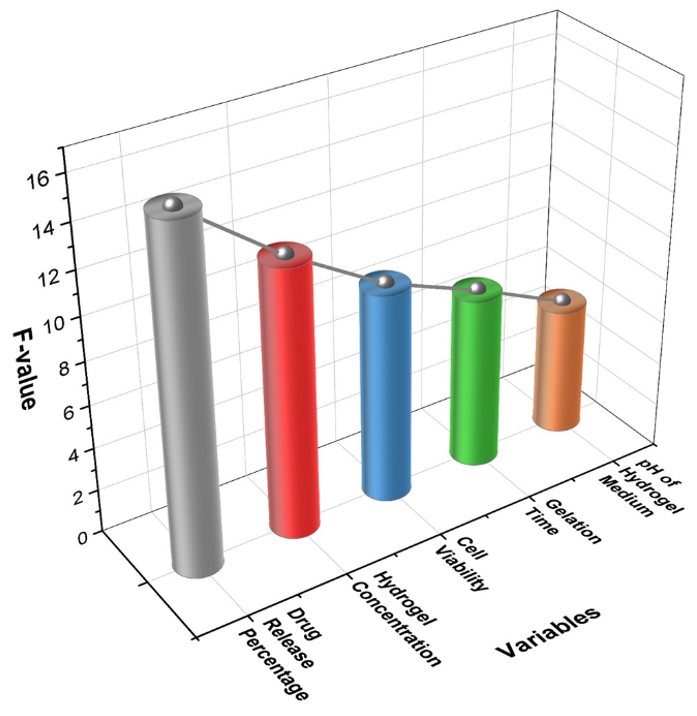
Figure 1. The outcome of the ANOVA Test
Tukey’s Post Hoc Test
Tukey’s Post Hoc Test analyzed Thermo-Reversible Hydrogel Systems for Customized Drug Release in CPD Treatment. Drug release showed significant differences (p< 0,05), while cytotoxicity remained non-significant, confirming biocompatibility. Higher concentrations led to increased drug release without affecting cell viability. Where r the Studentized range statistic from Tukey’s table is, MSwithin is ANOVA's mean square error, and m is sample size in equation (3).
![]()
The Tukey’s Post Hoc Test analyzed drug release profiles and cytotoxicity across different concentrations (1 mg/mL,5 mg/mL,and 10 mg/mL). Significant differences were observed in drug release, with mean differences of 12,5 (p = 0,017), 25,8 (p = 0,002) and 13,3 (p =0,012) for 1 mg/mLvs.5 mg/mL,1 mg/mLvs.10 mg/Ml and 5 mg/mLvs.10 mg/mL, respectively. In contrast, cytotoxicity showed no significant differences, with p-values of 0,315, 0,087 and 0,272. This suggests that while drug release significantly varies with concentration, cytotoxicity remains unaffected, indicating biocompatibility across the tested concentrations. Mean Difference (MD) shows the effect size between groups, Standard Error (SE) measures variability, and p-value (<0,05) indicates statistical significance, confirming whether observed differences are meaningful or due to random chance in hypothesis testing. Table 5 displays Tukey's post hoc test results for drug release and Cytotoxicity. Figure 2 Illustrate Tukey's Post Hoc Test result.
|
Table 5. Tukey’s Post Hoc Test Results for Drug Release and Biocompatibility & Cytotoxicity |
|||
|
Comparison Groups |
Mean Difference (MD) |
Standard Error (SE) |
p-Value |
|
Drug Release Profiles |
|||
|
1 mg/mLvs.5 mg/mL |
12,5 |
3,2 |
0,017 |
|
1 mg/mLvs.10 mg/mL |
25,8 |
3,5 |
0,002 |
|
5 mg/mLvs.10 mg/mL |
13,3 |
2,9 |
0,012 |
|
Biocompatibility & Cytotoxicity |
|||
|
1 mg/mLvs.5 mg/mL |
1,2 |
1,1 |
0,315 |
|
1 mg/mLvs.10 mg/mL |
2,5 |
1,4 |
0,087 |
|
5 mg/mLvs.10 mg/mL |
1,3 |
1,2 |
0,272 |
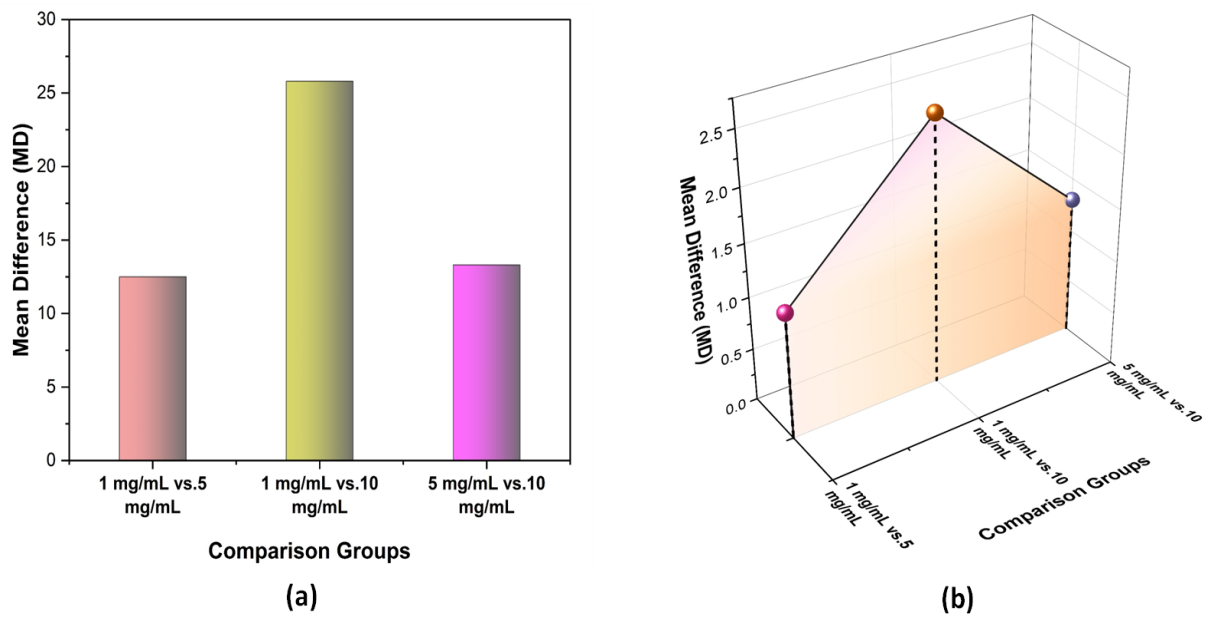
Figure 2. Illustrate the Tukey’s Post Hoc Test Result (a) Drug Release Profiles and (b)Biocompatibility & Cytotoxicity
DISCUSSION
Thermo- reversible hydrogel systems offer a customizable drug release approach for CPD treatment. These hydrogels enable temperature-sensitive drug delivery, ensuring sustained release at the disease site, reducing side effects, improving efficacy, and enhancing patient compliance for better periodontal health management. The IVDRP of DOX -loaded hydrogels over 72 hours demonstrates a concentration-dependent release. Higher DOX concentrations (10 mg/mL) exhibit a faster and more complete release (98,6 % at 72 hours) compared to lower concentrations (85,3 % for 1 mg/mL). The release rate increases significantly within the first 24 hours, stabilizing after 48 hours. The MTT assay results indicate a dose-dependent decrease in cell viability for both HGFs and PDL cells upon drug treatment. At the highest concentration (10 mg/mL), viability dropped to 92,8 % forHGFs and 91,3 % forPDLcells, compared to controls (98,6 % 98,3 %,). This suggests potential cytotoxic effects at higher doses. The ANOVA results indicate significant differences across hydrogel system variables. Drug release percentage shows the highest 15,67, < 0,001), suggesting a strong effect of experimental conditions. Hydrogel concentration also exhibits a notable influence (F = 12,32, p = 0,002). Cell viability (F = 9,65, p = 0,004) and gelation time (F = 7,89, p = 0,011) display statistical significance, highlighting their variability. The pH of the hydrogel medium has the lowest F value (5,87, p = 0,019), yet remains significant. These findings emphasize key factors affecting hydrogel performance. The findings of the Tukey's post hoc test show that the drug release profiles of the tested concentrations differ significantly. The 10 mg/mL group exhibited the highest release, with significant differences compared to both 1 mg/mL (MD = 25,8, p = 0,002) and 5 mg/mL (MD = 13,3, p = 0,012). In contrast, cytotoxicity analysis showed no statistically significant differences in cell viability across groups (p > 0,05), suggesting that increased drug concentrations do not notably impact biocompatibility, despite enhanced drug release at higher concentrations.
CONCLUSIONS
Thermo-reversible hydrogel systems offer a customizable drug release approach for CPD treatment. These hydrogels transition between sol and gel states, enabling sustained drug delivery. This enhances therapeutic efficacy, reduces dosing frequency, and improves patient compliance were promising solution for periodontal therapy. The potential of thermo-reversible hydrogels for controlled DOX delivery in periodontal therapy. Higher drug concentrations (10 mg/mL) led to rapid release within 24 hours, while lower concentrations (1 mg/mL and 5 mg/mL) ensured sustained release up to 72 hours. Cytotoxicity tests confirmed hydrogel biocompatibility (p>0,05) with HGFs and PDL cells. These results highlight the efficacy of PNIPAM-based hydrogels as localized, adjustable drug delivery systems, making them promising candidates for CPD treatment. Higher DOX concentrations (10 mg/mL) showed faster and more complete drug release, reaching 98,6 % at 72 hours, indicating enhanced drug availability and potential effectiveness for sustained therapeutic applications. The MTT assay shows high cell viability for HGFs and PDL cells, with control values of 98,6 % ± 1,8 and 98,3 % ± 2,0, respectively. Even at 10 mg/mL, viability remains above 91 %, indicating minimal toxicity. The ANOVA results show significant effects for all variables. Drug release percentage (F = 15,67, p < 0,001) has the strongest impact, followed by hydrogel concentration (F = 12,32, p = 0,002). Cell viability (F = 9,65, p = 0,004) also remains crucial for performance. Tukey’s test showed significant differences in drug release: 1 mg/mL vs.5 mg/mL (12,5 % p=0,017), 1 mg/mL vs.10 mg/mL (25,8 %, p=0,002), and 5 mg/mL vs.10 mg/mL (13,3 % p=0,012). Cytotoxicity differences were non-significant (p>0,05). Thermo-reversible hydrogel systems may face challenges such as limited drug loading capacity, potential variations and stability issues in gelation temperature affecting drug release. Long-term biocompatibility and patient-specific responses require further evaluation. Future scope can focus on optimizing hydrogel formulations for controlled release, improving mechanical stability, and integrating personalized medicine approaches using biomaterials tailored to individual periodontal conditions.
BIBLIOGAPHIC REFERENCES
1. Ayala-Ham A, López-Gutierrez J, Bermúdez M, Aguilar-Medina M, Sarmiento-Sánchez JI, López-Camarillo C, Sanchez-Schmitz G, Ramos-Payan R. Hydrogel-based scaffolds in oral tissue engineering. Frontiers in Materials. 2021 Jul 28;8:708945. https://doi.org/10.3389/fmats.2021.708945.
2. Shi J, Yu L, Ding J. PEG-based thermosensitive and biodegradable hydrogels. Actabiomaterialia. 2021 Jul 1;128:42-59. https://doi.org/10.1016/j.actbio.2021.04.009.
3. Chiriac AP, Ghilan A, Neamtu I, Nita LE, Rusu AG, Chiriac VM. Advancement in the biomedical applications of the (nano) gel structures based on particular polysaccharides. Macromolecular bioscience. 2019 Sep;19(9):1900187. https://doi.org/10.1002/mabi.201900187.
4. Pandey M, Choudhury H, Abdul-Aziz A, Bhattamisra SK, Gorain B, Carine T, Wee Toong T, Yi NJ, Win Yi L. Promising drug delivery approaches to treat microbial infections in the vagina: A recent update. Polymers. 2020 Dec 23;13(1):26. https://doi.org/10.3390/polym13010026.
5. Hafezi M, Nouri Khorasani S, Zare M, EsmaeelyNeisiany R, Davoodi P. Advanced hydrogels for cartilage tissue engineering: recent progress and future directions. Polymers. 2021 Nov 30;13(23):4199. https://doi.org/10.3390/polym13234199.
6. Yu J, Park SA, Kim WD, Ha T, Xin YZ, Lee J, Lee D. Current advances in 3D bioprinting technology and its applications for tissue engineering. Polymers. 2020 Dec 11;12(12):2958. https://doi.org/10.3390/polym12122958.
7. Li J, Chen G, Xu X, Abdou P, Jiang Q, Shi D, Gu Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regenerative biomaterials. 2019 Jun;6(3):129-40. https://doi.org/10.1093/rb/rbz022.
8. Wang F, Chen J, Liu J, Zeng H. Cancer theranostic platforms based on injectable polymer hydrogels. Biomaterials Science. 2021;9(10):3543-75. https://doi.org/10.1039/D0BM02149K.
9. Wang B, Booij-Vrieling HE, Bronkhorst EM, Shao J, Kouwer PH, Jansen JA, Walboomers XF, Yang F. Antimicrobial and anti-inflammatory thermo-reversible hydrogel for periodontal delivery. Actabiomaterialia. 2020 Oct 15;116:259-67. https://doi.org/10.1016/j.actbio.2020.09.018.
10. Wang B, Wang J, Shao J, Kouwer PH, Bronkhorst EM, Jansen JA, Walboomers XF, Yang F. A tunable and injectable local drug delivery system for personalized periodontal application. Journal of Controlled Release. 2020 Aug 10;324:134-45. https://doi.org/10.1016/j.jconrel.2020.05.004.
11. Tang G, Tan Z, Zeng W, Wang X, Shi C, Liu Y, He H, Chen R, Ye X. Recent advances of chitosan-based injectable hydrogels for bone and dental tissue regeneration. Frontiers in Bioengineering and Biotechnology. 2020 Sep 17;8:587658. https://doi.org/10.3389/fbioe.2020.587658.
12. Ciolacu DE, Nicu R, Ciolacu F. Cellulose-based hydrogels as sustained drug-delivery systems. Materials. 2020 Nov 21;13(22):5270. https://doi.org/10.3390/ma13225270.
13. Şenel S, Özdoğan AI, Akca G. Current status and future of delivery systems for prevention and treatment of infections in the oral cavity. Drug Delivery and Translational Research. 2021 Aug;11:1703-34.https://doi.org/10.1007/s13346-021-00961-2.
14. Vasile C, Pamfil D, Stoleru E, Baican M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules. 2020 Mar 27;25(7):1539. https://doi.org/10.3390/molecules25071539.
15. Gomez-Florit M, Pardo A, Domingues RM, Graça AL, Babo PS, Reis RL, Gomes ME. Natural-based hydrogels for tissue engineering applications. Molecules. 2020 Dec 11;25(24):5858. https://doi.org/10.3390/molecules25245858.
FINANCING
None.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Jamuna KV, Abhishek Kumar Mishra, Shakti Bedanta Mishra.
Data curation: Jamuna KV, Abhishek Kumar Mishra, Shakti Bedanta Mishra.
Formal analysis: Jamuna KV, Abhishek Kumar Mishra, Shakti Bedanta Mishra.
Drafting - original draft: Jamuna KV, Abhishek Kumar Mishra, Shakti Bedanta Mishra.
Writing - proofreading and editing: Jamuna KV, Abhishek Kumar Mishra, Shakti Bedanta Mishra.
ANNEXES
|
poly(N-isopropyl acrylamide) |
PNIPAM |
|
human gingival fibroblasts |
HGFs |
|
periodontal ligament |
PDL |
|
Chronic periodontal disease |
CPD |
|
doxycycline |
DOX |
|
lipoxin A4 |
LXA4 |
|
polylactic-co-glycolic acid |
PLGA |
|
chitosan |
CS |
|
In Vitro Drug Release Profile |
IVDRP |
|
Analysis of Variance |
ANOVA |