doi: 10.56294/mw2023148
ORIGINAL
Stem Cell-Based Cartilage Engineering Approaches for Osteochondral Defect Regeneration and Functional Tissue Repair
Enfoques de ingeniería de cartílago basados en células madre para la regeneración de defectos osteocondrales y la reparación funcional de tejidos
Uma Bhardwaj1 ![]() *, Puspak Samal2
*, Puspak Samal2 ![]() , Malathi H3
, Malathi H3 ![]()
1Noida International University, Department of Biotechnology and Microbiology, Greater Noida, Uttar Pradesh, India.
2IMS and SUM Hospital, Siksha ‘O’ Anusandhan (deemed to be University), Department of Orthopaedics, Bhubaneswar, Odisha, India.
3JAIN (Deemed-to-be University), Department of Biotechnology and Genetics, Bangalore, Karnataka, India.
Cite as: Bhardwaj U, Samal P, Malathi H. Stem Cell-Based Cartilage Engineering Approaches for Osteochondral Defect Regeneration and Functional Tissue Repair. Seminars in Medical Writing and Education. 2023; 2:148. https://doi.org/10.56294/mw2023148
Submitted: 07-10-2022 Revised: 12-01-2023 Accepted: 14-04-2023 Published: 15-04-2023
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding author: Uma Bhardwaj *
ABSTRACT
Osteochondral defects offer important concerns in orthopedic medicine suitable to the restricted self-curing ability of cartilage. Stem cell-based cartilage engineering has emerged as a capable method for OC defect regeneration and functional tissue repair. Research explores the SDSCs-based cartilage engineering approaches for OC defect regeneration and functional tissue repair. Rabbit knee joints are used to separate SDSCs, which are then grown to passage 5 and added to a fibrin glue- PGA scaffold. The designed premature cartilage constructions are transplanted into rabbit femoral condyle defects following 50 days of in vitro stimulation in a growth factor-enriched environment. Research evaluated two groups: the Construct Group, where SDSC-engineered premature cartilage is implanted with Collagraft as a bone alternate, and the Empty Group, where defects are left untreated. The data is analyzed using statistical evaluation performed with SPSS software. Histological analysis showed that SDSCs effectively underwent chondrocyte differentiation, merging with native cartilage and generating collagen III and sulfated GAG. While the Empty Group generated fibrocartilage, indicating worse regeneration, the Construct Group showed uniform hyaline-like cartilage with strong collagen III and GAG expression and no detectable collagen I or macrophages at eight months after implantation. These results demonstrate the viability of using SDSC-based synthetic cartilage to heal OC defects.
Keywords: SDSCs; OC Defect; Regeneration; Tissue Repair; Fibrin Glue- PGA; Collagraft.
RESUMEN
Los defectos osteocondrales plantean importantes problemas en medicina ortopédica debido a la limitada capacidad de autocuración del cartílago. La ingeniería del cartílago basada en células madre se ha revelado como un método capaz de regenerar defectos OC y reparar tejidos funcionales. La investigación explora los enfoques de ingeniería del cartílago basados en SDSC para la regeneración de defectos OC y la reparación del tejido funcional. Se utilizan articulaciones de rodilla de conejo para separar las SDSC, que luego se cultivan hasta el paso 5 y se añaden a un andamio de cola de fibrina y PGA. Las construcciones de cartílago prematuro diseñadas se trasplantan en defectos del cóndilo femoral de conejo tras 50 días de estimulación in vitro en un entorno enriquecido con factores de crecimiento. La investigación evaluó dos grupos: el grupo de construcción, en el que se implanta cartílago prematuro diseñado con SDSC y Collagraft como sustituto óseo, y el grupo vacío, en el que los defectos se dejan sin tratar. Los datos se analizaron mediante una evaluación estadística realizada con el programa SPSS. El análisis histológico demostró que las SDSC experimentaron efectivamente la diferenciación de condrocitos, fusionándose con el cartílago nativo y generando colágeno III y glicosaminoglicanos sulfatados. Mientras que el grupo vacío generó fibrocartílago, lo que indica una peor regeneración, el grupo constructo mostró un cartílago uniforme de aspecto hialino con una fuerte expresión de colágeno III y glicosaminoglicanos y sin colágeno I ni macrófagos detectables a los ocho meses de la implantación. Estos resultados demuestran la viabilidad del uso de cartílago sintético basado en SDSC para la curación de defectos OC.
Palabras clave: SDSCs; Defecto OC; Regeneración; Reparación Tisular; Pegamento de Fibrina- PGA; Collagraft.
INTRODUCTION
The articular cartilage system of the body depends heavily on cartilage since hyaline cartilage absorbs concussions and stops bone abrasion. Articular cartilage’s natural structural properties make it incapable of regenerating following damage or in situations involving pathological disorders.(1) In orthopedics, the use of different kinds of stem cells for musculoskeletal examination along with the growth of new approaches for the change of cell-based therapies to medical has expanded significantly. Stem cell investigation in orthopedics has covered from nascent stem cells and iPSCs to various kinds of mature stem cells, frequently known as MSCs.(2) Conservative nonsurgical treatment and pharmaceutical interventions for OA helps in reducing pain in the initial phases of the illness’s progression, but they cannot stop cartilage degradation. Steroid and nonsteroidal anti-inflammatory medications, such as corticosteroids and HA injections, provide symptoms relief but they have little effect on the disease’s development.(3) The glassy coating that covers the articular exterior of bone ends is called articular cartilage. It facilitates smooth and painless action of joints by decreasing bone-to-bone resistance and cushioning external shocks. These cells within the cartilage create the ECM mechanism, including proteoglycans, collagen, and hyaluronic acid, providing cartilage its automatic features.(4) To achieve more consistent hyaline cartilage regeneration, modern approaches use in vitro-expanded cells to enlarge organoids of cell phenotypes, dosing, and populations. The MSCs have been carefully investigated as cell causes for cartilage cell production due to extensive in vitro growth abilities, accessibility, chondrogenic potential, and ancestry ability.(5)
The low regeneration ability of cartilage makes tissue lesions clinically significant. The primary challenge with existing healing techniques is their insufficiency to fully restore the tissue’s function or return it to healthy condition. Even though cartilage was once thought to have a simple construction, replicating its highly stratified structure has proven difficult.(6) Cartilage abnormalities caused by trauma or joint detection hinder everyday movements like walking or running. These lesions quickly progress to OA, resulting in the total loss of articular function and the consequent requirement for joint replacement, since cartilage has a restricted ability for self-curing. The limits of conventional surgical methods for cartilage regeneration have contributed to the growth of cell-based therapies in recent decades.(7) The research described how OC tissues were engineered in vivo using unified gallows with a depth-varying pore design and stone setting.(8) The trilayer gallows was created to encourage the contributor cells to continue differentiating into cartilage cells while promoting bone shape by attracting internal cells. These partially cell-loaded scaffolds ingrained in vivo generated ostecochondral tissue with a lubricant-rich surface.
The investigation offered an overview of the mechanical characteristics, microscale structure, cell type, scaffold material, manufacturing techniques, and scaffold stimuli of both used in separate and nonstop gradient OC tissue scaffolds.(9) The capacity of 3D printing to precisely manipulate scaffold pore shape made it a specific focus in gradient scaffold fabrication. The method improved scaffold shape enhanced mechanical qualities, and Physical cues created inside the scaffold-bioreactor to improve tissue rejuvenation, along with the request of computer modeling in OC tissue engineering.
Research used 3D knee joints created from medicinal imaging and mechano-guideline theory to anticipate the automatic characteristics of an ideal gibbet for cartilage rejuvenation.(10) It was expected that a scaffold with the best mechanical qualities would produce more cartilage tissue than one without.
Research provided the latest advancements in 3D bio printing for clinical use, emphasizing the ability to create customized 3D-printed skin, bone, and cartilage utilizing the individual’s cells.(11) Important translational aspects were also covered, including the necessity of ensuring the printed tissue with the individual’s vascular system, the creation of bio-friendly inks, and the difficulties in determining a biologically appropriate cell count.
Research described that the quickly evolving system of bone tissue engineering is interconnected with many other disciplines, like genetics, clinical medicine, mechanical engineering, and material science.(12) Scaffolds were user-generated settings helpful to tissue regeneration and repair, aimed to restore and enhance bone tissue function. Oral regenerative therapy techniques and materials were similar to those typically employed in bone tissue engineering.
Research evaluated MSC treatments for cartilage restoration. A modest database was created using published clinical and in vivo research.(13) The individual-explicit cartilage healing following MSC treatment was shown in the research. The method is modified for use in various therapeutic purposes and used to find and examine other crucial characteristics involved in MSC-induced cartilage healing.
The system is intended to promote MSC chondrogenic development as cell sheets to create transplantable hyaline-like cartilage structures.(14) The 3D MSC sheets were created by chondrogenically inducing them and taking advantage of impulsive post-detachment Cellular Membrane Constriction. The findings indicated the chondrogenic differentiation of 3D MSC membranes to hyaline cartilage in vitro through structural changes and post-contraction cytoskeletal rearrangement.
Research evaluated the genetic action that encourages intention cells to state novel and suitable tissue, such as many synthetic bio absorbable polymers.(15) The estimation was made that a conventional scaffold was the incorrect strategy and that tissue-engineering patterns that were made to mimic the target cells’ place have a far higher chance of success.
Research estimated an individual adipose-resultant MSC-based medicinal micro automaton method for knee cartilage rejuvenation.(16) The micro automaton method was made up of an automaton body that could sustain MSCs for 3D targeting, and an attraction to hold the micro automaton to the injured cartilage. Every constituent was developed and built with convenience for patients and remedial professionals in mind, as well as scientific security. The micro automaton method effectiveness was evaluated in a rabbit knee cartilage flaw model to receive clinical trial approval. Research objective is to analyze the effectiveness of SDSCs in cartilage engineering for OC defect repair. Research’s specific objective is to assess the ability of SDSCs combined with a fibrin glue- PGA scaffold to stimulate chondrocyte differentiation, cartilage production, and tissue healing in a rabbit model. Research compares the outcome of SDSC-engineered cartilage to untreated defects to assess cartilage quality and regeneration.
METHOD
Research examined SDSC-based cartilage regeneration using cell isolation, scaffold fabrication, and in vivo implantation in a rabbit model. Histological and biochemical analyses assessed extracellular matrix composition, while ANOVA and paired t-tests evaluated statistical differences. The approach ensured a systematic evaluation of SDSC-based constructs for OC defect repair, highlighting their regenerative potential.
Cell isolation and culture
SDSCs are extracted from rabbit knee joints utilizing enzymatic digestion. The synovial tissue is extracted in a sterile environment, cut into minute fragments, and treated with collagenase type I to break down cells. The SF specimens are diluted an expansion medium and placed on Petri plates. After 3-4 days, the civilization medium is restored to eliminate inactive cells. Cells are cultivated in DMEM-LG including 10 % FBS, 1 ng/ml bFGF, 1 % C₅H₁₀N₂O₃, and 1 % C₁₉H₁₉N₂O₅S- C₂₀H₃₉N₇O₁₂. The plates are cultivated at 37°C with 5 % moist CO2. The medium is adjusted twice per week until confluence, after remaining unaltered for the first three days. Non-adherent cells are removed by changing the media in sequence. MSCs are trypsinized and dished at 0,5 × 106 cells per flask once adherent cells reached 80 % confluence. The medium is distorted the next day and thereafter all days. A pre-differentiation step is done before planting the cells in collagen sponges during the third passage. SF-MSCs are cultured in DMEM-HG (Gibco) including C₃H₃NaO₃, 1 % penicillin-streptomycin, and chondrogenic components such as C₅H₉NO₂, L-ascorbic acid-2- H₃PO₄, and C22H29FO5.
Scaffold Fabrication and Cell Seeding
Degradable PGA scaffolds are struck into plates and cleaned with C₂H₄O. These discs are then engrossed in 100 % C₂H₅OH, 70 % C₂H₅OH, and Phosphate-Buffered Saline (PBS). To prepare a centrifuge tube, add 150 mL fibrinogen140 mL PBS containing cells, 5 mL C21H29N3O8S2, and 5 mL CaCl2 consecutively. The SDSC-gelis pipette onto a PGA solution in a Petri plates. This technique yielded 56 fibrin-PGA combined with 2,6 106 cells per scaffold, representing a first Seeding intensity of 100 106 cells/mL. The dish with constructs is placed in an incubator for 10 minutes. The creations are then completely covered with media.
After 1 hour, the medium is restored with a biologically distinct solution added with a proliferative development feature cocktail. The cell-fibrin-PGA constructions are placed in a rotating bioreactor with chemically specified media and a differentiate growth factor cocktail for 28 days. The bioreactor revolving velocity is modified to keep the rising assembles floating at the revolving speed. At days 0, 3, 15, and 31, 44 tissue constructions are collected for analysis purposes. Another 12 one-month constructions are used for in vivo implantation.
Implementation in vivo
Following common anesthesia, a Spherical flaw is made in the patellar articular cartilage. The dimension of the fault is placed at 4,8 mm. The deepness is discovered to be similar to the height of the cell development typically measured at 4-5 mm and attained the centerlayer of the subchondral bone. The cell is carefully inserted into the flaw, the Teflon plate is detached, and the surgeon flattened the apex of the build with handle. No spaces are applied to the insert solution. After cleansing and dressing the injury, the knee is embedded with a shed for one week. Rabbits are executed at 3, 6, and 12 weeks to verify the early rejuvenation phase through histological analysis. Following optimistic consequences from this trial, three knees are retrieved from all of the two groups and histologically examined.
Data splitting
The rabbit femoral condyle injury model is utilized to evaluate cartilage regeneration. The research included two groups: an experimental and an empty group.
Experimental group
The Construct Group had SDSC-engineered preterm cartilage implantation in combination with Collagraft as a bone replacement.
Empty group
The Empty Group acted as a negative control, with OC lesions left untreated to determine natural healing.
This comparison demonstrates the efficacy of SDSC-based cartilage constructions for OC defect healing.
Histological analysis
Specimens are set in 4 % (CH₂O)ₓ, decalcified using 0,5 M EDTA, and implanted in paraffin wax. For immune-histo-chemistry, slices are pretreated with 0,4 mg/mL C26H39N7O13S2 for 10 minutes at room temperature. The peroxidases are extinguished with 3 % H₂O₂ in methanol for 20 minutes at room temperature. The slices are then treated with a polyclonal antibody beside type II collagen at room temperature for 1 hour. The VECTASTAIN ABC reagent is used for immune-staining is produced in 3,3’-diaminobenzidine and 0,02 % H2O2 before being counterstained with hematoxylin.
Histological scoring
The histological score is based on cartilage thickness (max 2), matrix staining (max 3), collagen composition, cell morphology (max 4), and surface regularity (max 3). Blinded evaluations are performed by two independent orthopedic surgeons to ensure an objective assessment of SDSC-based cartilage regeneration and integration into host tissue.
Biochemical analysis
Cartilage samples from each group are processed for 6 hours at 60°C using 125 µg/mL papain in PBE buffer with 10 mmol/L cysteine. sGAG concentration is determined utilizing DMMB dye and a Spectronic BioMate 3 Spectrophotometer. Collagen composition is assessed using C₆H₉NO₃ tests, and DNA content indicated cell density is quantified using a Quant-iTPicoGreendsDNA Assay kit and a CytoFluor Series 4000 fluorometer. These biochemical assays allowed for the exact measurement of extracellular matrix components, demonstrating the quality of cartilage regeneration.
Statistical analysis
The statistics are evaluated using SPSS 27 software. The data is analyzed using various tests, including ANOVA and paired t-test. ANOVA is used to examine biochemical and histological changes between the Construct and Empty groups. Paired t-tests are used to examine within-group differences over time.
RESULTS
Research estimates the efficiency of SDSC-based cartilage engineering for repairing OC defects. The outcomes are evaluated using histological analysis that looks at extracellular and tissue morphology matrix, as well as biochemical analysis that calculates sGAG and collagen levels. Statistical analysis, including paired t-tests and one-way ANOVA, exposed major differences between the Empty Group and the Construct Group.
Paired t-Test
The paired t-test contrasts the value of cartilage regeneration in the Empty Group and Construct Group. It explores whether SDSC-based therapy leads to considerable enhancements in cartilage regeneration. Figure 1 and table 1 illustrate the assessment of variables.
|
Table 1. Evaluation of t-test |
|||||
|
Comparison |
Mean Difference (MD) |
Standard Deviation (SD) |
Standard Errors (SE) |
t- Statistic |
p-value |
|
Collagen III Expression |
2,50 |
1,12 |
0,56 |
4,46 |
0,001 |
|
GAG Expression |
1,80 |
0,98 |
0,49 |
3,67 |
0,004 |
|
Overall Cartilage Quality |
3,20 |
1,05 |
0,52 |
6,15 |
0,0002 |
MD-Mean Difference, SD- Standard deviation, SE- standard errors
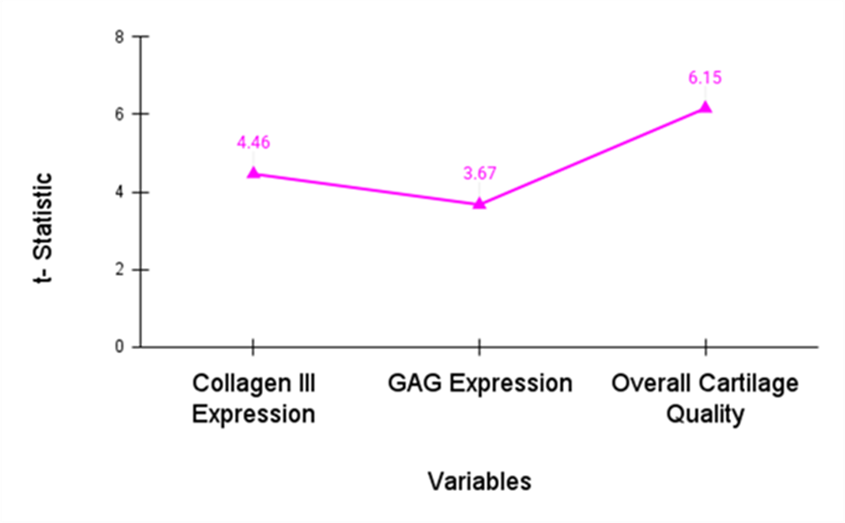
Figure 1. Estimation of t-statistic
The results of the paired t-test show that the Construct Group had considerably better cartilage regeneration than the Empty Group. The Construct Group had improved GAG expression (p=0,004), overall cartilage quality (p=0,002), and collagen III expression (p=0,001), representing the effectiveness of SDSC-based therapy for OC desert repair. A p-value of less than 0.05 shows SDSC-based therapy’s competence in promoting cartilage regeneration and remedial OC defects when compared to untreated lesions.
ANOVA Test
An ANOVA is utilized to evaluate the average of different groups to perceive whether there are any major distinctions. ANOVA is employed to evaluate cartilage regeneration results like GAG expression, collagen III expression, and overall cartilage value between the Empty Group and the Construct Group. It examines if SDSC-based cartilage engineering greatly develops regeneration compared to untreated defects. Table 2 represents the statistical analysis. Figure 2 shows the outcomes of the analysis.
|
Table 2. Statistical analysis of ANOVA |
||||
|
Variables |
SS |
MS |
F-value |
P -value |
|
Collagen III Expression |
25,50 |
12,75 |
5,43 |
0,001 |
|
GAG Expression |
18,40 |
9,20 |
4,12 |
0,004 |
|
Overall Cartilage Quality |
30,00 |
15,00 |
6,00 |
0,0002 |
|
Within cohort |
40,00 |
4,00 |
|
|
|
Between-cohort |
74,49 |
24,97 |
|
|
|
Total |
114,90 |
|
|
|
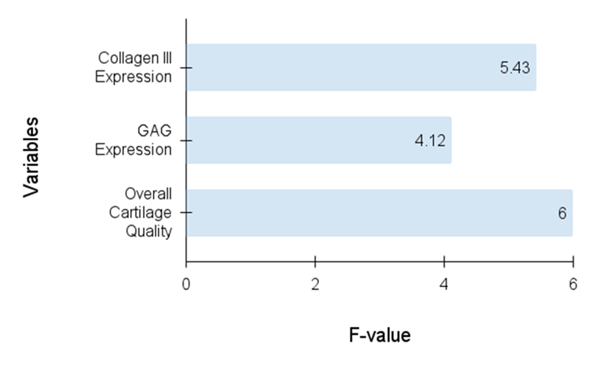
Figure 2. Evaluation of F-Value
SS- Sum of Square, MS-Mean Square
The results show significant differences in cartilage regeneration between the Construct Group and the Empty Group. Collagen III expression (p = 0,001), GAG expression (p = 0,004), and overall cartilage quality (p = 0,0002) were all considerably greater in the construct group. The between-cohort variation (SS = 74,49) was significantly greater than the within-cohort variance (SS = 40,00), demonstrating the efficacy of SDSC-based therapy in promoting cartilage regeneration.
Histology analysis
The research of tissues’ microscopic formation is called histology. Tissue samples are examined under a microscope to determine their organization, composition, and function. Histological analysis is used in the investigation to assess the value of cartilage regeneration by comparing the expression of markers like GAG and collagen III in the Empty Group and the Construct Group, providing insights into the efficiency of SDSC-based treatment for OC defect repair. Table 3 demonstrates the histological analysis.
|
Table 3. Histology Evaluation |
|||
|
Parameter |
Construct group |
Empty Group |
p-value |
|
Cartilage Thickness m |
42025 |
26030 |
<0,001 |
|
Safranin O Staining |
3,80,3 |
2,10,4 |
<0,01 |
|
Collagen II Staining |
4,20,4 |
1,50,5 |
<0,001 |
|
Collagen I Staining |
0,20,1 |
3,90,2 |
<0,001 |
|
Cell Morphology |
4,50,3 |
2,00,5 |
<0,001 |
|
Surface Regularity |
4,30,2 |
1,80,4 |
<0,001 |
The histology investigation discovered that the Construct Group overcomes the Empty Group. The Construct Group had more cartilage Safranin O staining, thickness, and Collagen II staining while having less Collagen I staining, representing enhanced cartilage production. In addition, the surface uniformity and Construct Group had greater cell morphology, signifying the effectiveness of SDSC-based therapy in promoting cartilage regeneration.
Biochemical Analysis
The Biochemical analysis includes examining biochemical markers in tissues to resolve their function and composition. The research quantifies cartilage regeneration using significant indicators like GAG and collagen type II. This examination confirms the efficiency of SDSC-based therapy by detecting biochemical changes in the cartilage, such as superior GAG production and collagen II expression, representing enhanced cartilage regeneration in the Construct Group evaluated to the Empty Group. Table 4 shows the biochemical analysis evaluation.
|
Table 4. Biochemical analysis |
|||
|
Parameter |
Construct Group |
Empty Group |
p-value |
|
sGAG (g/mg) |
38,42,5 |
12,11,8 |
<0,001 |
|
Collagen III (%) |
48,73,2 |
18,51,18 |
<0,01 |
|
Collagen I (%) |
2,50,8 |
42,13,5 |
<0,001 |
Biochemical enquiry shows significant developments in the Construct Group as estimated to the Empty Group. The Construct Group had considerably better sGAG levels and Collagen III expression, but lower Collagen I expression, demonstrating enhanced cartilage regeneration. These outcomes support the efficiency of SDSC-based therapy in improving cartilage repair and boosting optimum biochemical indicators for OC desert regeneration.
DISCUSSION
Research is required to evaluate the effectiveness of SDSC-based therapy for cartilage regeneration. The results were analyzed using ANOVA, paired t-tests, histology, and biochemical examination. The paired t-test demonstrated substantial developments in GAG expression (p = 0,004), collagen III expression (p = 0,001), and overall cartilage quality (p = 0,0002) in the Construct Group over the Empty Group. ANOVA demonstrates important differences across groups (p < 0,05), representing enhanced cartilage regeneration. Construct Group, histological inquiry exposed thicker cartilage, advanced collagen II staining and Safranin O, and better surface regularity (p < 0,001). Biochemical investigation showed improved collagen III expression and sGAG levels, with less collagen I present, representing enhanced cartilage composition (p < 0,001). These data support the effectiveness of SDSC-based therapy in promoting OC defect repair and, the presentation of it’s potential as a regenerative technique for improving cartilage value and function.
CONCLUSION
The SDSC-based cartilage engineering has important effects for restoring hyaline cartilage and restoring joint function in OC anomalies. This method increases long-term curing, reduces scar tissue, and improves cartilage and subchondral bone regeneration, decreasing the requirement for invasive processes and expanding joint health. The research aimed to evaluate the capability of SDSC-engineered cartilage to support cartilage regeneration in a rabbit model with OC defects. The outcomes demonstrate that the Construct Group had significantly higher collagen III expression, GAG synthesis, cartilage thickness, and overall cartilage value compared to the Empty Group. Histological and biochemical studies validated the Construct Group’s improved cartilage regeneration. However, the research had drawbacks, such as the use of a particular animal model. Further research should focus on developing scaffold materials, performing human clinical tests, and extending the research era to assess long-term effectiveness. These developments lead to the extensive use of SDSC-based cartilage engineering in clinical therapies for OC irregularities.
REFERENCES
1. Neybecker P, Henrionnet C, Pape E, Mainard D, Galois L, Loeuille D, Gillet P, Pinzano A. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem cell research & therapy. 2018 Dec;9:1-5. https://doi.org/10.1186/s13287-018-1071-2
2. Guilak F, Pferdehirt L, Ross AK, Choi YR, Collins K, Nims RJ, Katz DB, Klimak M, Tabbaa S, Pham CT. Designer stem cells: Genome engineering and the next generation of cell‐based therapies. Journal of Orthopaedic Research®. 2019 Jun;37(6):1287-93. https://doi.org/10.3390/ijms21176046
3. Liu Y, Wang M, Luo Y, Liang Q, Yu Y, Chen F, Yao J. Enhancing stem cell therapy for cartilage repair in osteoarthritis—a hydrogel focused approach. Gels. 2021 Dec 14;7(4):263. https://doi.org/10.3390/gels7040263
4. Nam Y, Rim YA, Lee J, Ju JH. Current therapeutic strategies for stem cell‐based cartilage regeneration. Stem cells international. 2018;2018(1):8490489. https://doi.org/10.1155/2018/8490489
5. Thorp H, Kim K, Kondo M, Maak T, Grainger DW, Okano T. Trends in articular cartilage tissue engineering: 3D mesenchymal stem cell sheets as candidates for engineered hyaline-like cartilage. Cells. 2021 Mar 13;10(3):643. https://doi.org/10.3390/cells10030643
6. Stampoultzis T, Karami P, Pioletti DP. Thoughts on cartilage tissue engineering: A 21st century perspective. Current Research in Translational Medicine. 2021 Jul 1;69(3):103299. https://doi.org/10.1016/j.retram.2021.103299
7. Lopa S, Mondadori C, Mainardi VL, Talò G, Costantini M, Candrian C, Święszkowski W, Moretti M. Translational application of microfluidics and bioprinting for stem cell‐based cartilage repair. Stem cells international. 2018;2018(1):6594841. https://doi.org/10.1155/2018/6594841
8. Kang H, Zeng Y, Varghese S. Functionally graded multilayer scaffolds for in vivo osteochondral tissue engineering. Actabiomaterialia. 2018 Sep 15;78:365-77. https://doi.org/10.1016/j.actbio.2018.07.039
9. Zhang B, Huang J, Narayan RJ. Gradient scaffolds for osteochondral tissue engineering and regeneration. Journal of Materials Chemistry B. 2020;8(36):8149-70. https://doi.org/10.1039/D0TB00688B
10. Koh YG, Lee JA, Kim YS, Lee HY, Kim HJ, Kang KT. Optimal mechanical properties of a scaffold for cartilage regeneration using finite element analysis. Journal of tissue engineering. 2019 Feb;10:2041731419832133. https://doi.org/10.1177/2041731419832133
11. Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nature biomedical engineering. 2020 Apr;4(4):370-80. https://doi.org/10.1038/s41551-019-0471-7
12. Chocholata P, Kulda V, Babuska V. Fabrication of scaffolds for bone-tissue regeneration. Materials. 2019 Feb 14;12(4):568. https://doi.org/10.3390/ma12040568
13. Liu YY, Lu Y, Oh S, Conduit GJ. Machine learning to predict mesenchymal stem cell efficacy for cartilage repair. PLoS computational biology. 2020 Oct 7;16(10):e1008275. https://doi.org/10.1371/journal.pcbi.1008275
14. Thorp H, Kim K, Kondo M, Grainger DW, Okano T. Fabrication of hyaline-like cartilage constructs using mesenchymal stem cell sheets. Scientific reports. 2020 Nov 30;10(1):20869. https://doi.org/10.1038/s41598-020-77842-0
15. Williams DF. Challenges with the development of biomaterials for sustainable tissue engineering. Frontiers in bioengineering and biotechnology. 2019 May 31;7:127. https://doi.org/10.3389/fbioe.2019.00127
16. Go G, Jeong SG, Yoo A, Han J, Kang B, Kim S, Nguyen KT, Jin Z, Kim CS, Seo YR, Kang JY. Human adipose–derived mesenchymal stem cell–based medical microrobot system for knee cartilage regeneration in vivo. Science Robotics. 2020 Jan 22;5(38):eaay6626. https://doi.org/10.1126/scirobotics.aay6626
FINANCING
None.
CONFLICT OF INTEREST
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Uma Bhardwaj, Puspak Samal, Malathi H.
Data curation: Uma Bhardwaj, Puspak Samal, Malathi H.
Formal analysis: Uma Bhardwaj, Puspak Samal, Malathi H.
Research: Uma Bhardwaj, Puspak Samal, Malathi H.
Methodology: Uma Bhardwaj, Puspak Samal, Malathi H.
Project management: Uma Bhardwaj, Puspak Samal, Malathi H.
Resources: Uma Bhardwaj, Puspak Samal, Malathi H.
Software: Uma Bhardwaj, Puspak Samal, Malathi H.
Supervision: Uma Bhardwaj, Puspak Samal, Malathi H.
Validation: Uma Bhardwaj, Puspak Samal, Malathi H.
Display: Uma Bhardwaj, Puspak Samal, Malathi H.
Drafting - original draft: Uma Bhardwaj, Puspak Samal, Malathi H.
Writing: Uma Bhardwaj, Puspak Samal, Malathi H.
ANNEXES
|
Table 1. Acronym- Abbreviation |
|
|
Acronym |
Abbreviation |
|
SDCs |
Synovium-Derived Stem Cells |
|
PGA |
Polyglycolic Acid |
|
SPSS |
Statistical Package for the Social Sciences |
|
GAG |
Glycosaminoglycan |
|
iPSCs |
induced Pluripotent Stem Cells |
|
MSCs |
Mesenchymal Stem Cells |
|
OA |
Osteoarthritis |
|
HA |
hyaluronic acid |
|
ECM |
Extracellular Matrix |
|
OC |
Osteochondral |
|
3D |
3 dimensional |
|
DMEM-LG |
Dulbecco’s Modified Eagle Medium-Low Glucose |
|
FBS |
Fetal Bovine Serum |
|
bFGF |
Basic Fibroblast Growth Factor |
|
CO2 |
Carbon dioxide |
|
PBS |
Phosphate-Buffered Saline |
|
CaCl2 |
Calcium chloride |
|
SDSC |
Synovium-Derived Stem Cells |
|
EDTA |
Ethylenediaminetetraacetic Acid |
|
VECTASTAIN ABC |
Vector Staining Avidin-Biotin Complex |
|
sGAG |
Sulfated glycosaminoglycan |
|
DMMB |
dimethyl methylene blue |
|
DNA |
Deoxyribonucleic Acid |
|
ANOVA |
Analysis of Variance |
|
MD |
Mean Difference |
|
SD |
Standard Deviation |
|
SE |
Standard Errors |
|
C₅H₁₀N₂O₃ |
glutamine |
|
C₁₉H₁₉N₂O₅S- C₂₀H₃₉N₇O₁₂ |
penicillin-streptomycin |
|
C₃H₃NaO₃ |
sodium pyruvate |
|
C₃H₄O₃ |
pyruvate |
|
C₂H₅OH |
ethanol |
|
C21H29N3O8S2 |
thrombin |
|
H₃PO₄ |
phosphate |
|
C₁₈H₃₆ to C₅₀H₁₀₀ |
paraffin wax |
|
(CH₂O)ₓ |
paraformaldehyde |
|
C20H19ClN4 |
safranin O |
|
C₁₆H₁₄O₆ |
hematoxylin |
|
C₁₆H₁₈ClN₃S |
dimethylmethylene blue |
|
C₆H₉NO₃ |
hydroxyproline |
|
C₅H₉NO₂ |
proline |
|
C22H29FO5 |
dexamethasone |
|
C₆H₈O₆P |
L-ascorbic acid-2-phosphate |
|
C₂H₄O |
ethylene oxide |
|
C26H39N7O13S2 |
proteinase K |
|
H₂O₂ |
hydrogen peroxide |