doi: 10.56294/mw2023149
ORIGINAL
Therapeutic potential of stem cells for treating retinal ganglion cell degeneration in optic neuropathies
Potencial terapéutico de las células madre para tratar la degeneración de las células ganglionares de la retina en las neuropatías ópticas
Jamuna KV1 ![]() *,
Uma Bhardwaj2
*,
Uma Bhardwaj2 ![]() , Surjya Prakash S3
, Surjya Prakash S3 ![]()
1JAIN (Deemed-to-be University), Department of Forensic science. Bangalore, Karnataka, India.
2Noida International University, Department of Biotechnology and Microbiology. Greater Noida, Uttar Pradesh, India.
3IMS and SUM Hospital, Siksha ‘O’ Anusandhan (deemed to be University), Department of Neurology. Bhubaneswar, Odisha, India.
Cite as: Jamuna K, Bhardwaj U, Surjya PS. Therapeutic Potential of Stem Cells for Treating Retinal Ganglion Cell Degeneration in Optic Neuropathies. Seminars in Medical Writing and Education. 2023; 2:149. https://doi.org/10.56294/mw2023149
Submitted: 19-09-2022 Revised: 31-12-2022 Accepted: 03-03-2023 Published: 04-03-2023
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding Author: Jamuna KV *
ABSTRACT
Retinal ganglion cell (RGC) degeneration is a symbol of optic neuropathies, leading to progressive vision loss with limited therapeutic options. Stem cell-based medicines have gained recognition as a potential breakthrough strategy for neuro-protection and regeneration in such conditions. This research investigated the efficacy of Human Periodontal Ligament-Derived Stem Cells (PDLSCs) and the Extracellular Vesicles (EVs) of Optic Neuropathies in a rat model. PDLSCs were isolated, characterized, and transplanted either as whole cells or via their secreted EVs. The neuroprotective and regenerative effects were assessed at 15, 61, and 120 days post-injury through histological analysis, RGC survival counts, axonal regeneration assays, and functional restoration of visual pathways. Additionally, serum-deprived PDLSCs were used to evaluate their impact on neuroprotection compared to regular PDLSCs. PDLSC transplantation significantly enhanced RGC survival and reduced apoptosis for up to 120 days post-injury, with marked improvements observed at 15 and 61 days. Axonal regeneration along the optic nerve was evident, particularly at 120 days, demonstrating a sustained regenerative response. Similar neuroprotective effects were observed with serum-deprived PDLSCs, suggesting a potential role of paracrine signaling in their therapeutic action. However, transplantation of EVs isolated from PDLSCs failed to induce significant neuroprotection or axonal regeneration, indicating that direct cellular interaction can be essential for optimal therapeutic outcomes. These results support further exploration of PDLSCs as a viable cell therapy approach for neurodegenerative conditions affecting the optic nerve.
Keywords: Stem Cells; Retinal Ganglion Cell (RGC); Human Periodontal Ligament-Derived Stem Cells (PDLSCS); Optic Neuropathies; Extracellular Vesicles (EVS).
RESUMEN
La degeneración de las células ganglionares de la retina (CGR) es un símbolo de las neuropatías ópticas, que conduce a la pérdida progresiva de la visión con opciones terapéuticas limitadas.
Los medicamentos basados en células madre han ganado reconocimiento como una posible estrategia innovadora para la neuroprotección y la regeneración en tales condiciones. En esta investigación se estudió la eficacia de las células madre humanas derivadas del ligamento periodontal (PDLSC) y las vesículas extracelulares (EV) de las neuropatías ópticas en un modelo de rata. Las PDLSCs fueron aisladas, caracterizadas y trasplantadas como células enteras o a través de sus EVs secretadas. Los efectos neuroprotectores y regenerativos se evaluaron a los 15, 61 y 120 días tras la lesión mediante análisis histológicos, recuentos de supervivencia de CGR, ensayos de regeneración axonal y restauración funcional de las vías visuales. Además, se utilizaron PDLSC privadas de suero para evaluar su impacto en la neuroprotección en comparación con las PDLSC normales. El trasplante de PDLSC mejoró significativamente la supervivencia de las RGC y redujo la apoptosis hasta 120 días después de la lesión, observándose marcadas mejoras a los 15 y 61 días. La regeneración axonal a lo largo del nervio óptico fue evidente, sobre todo a los 120 días, lo que demuestra una respuesta regenerativa sostenida. Se observaron efectos neuroprotectores similares con PDLSCs privadas de suero, lo que sugiere un papel potencial de la señalización paracrina en su acción terapéutica. Sin embargo, el trasplante de EVs aisladas de PDLSCs no logró inducir una neuroprotección significativa o la regeneración axonal, lo que indica que la interacción celular directa puede ser esencial para obtener resultados terapéuticos óptimos. Estos resultados apoyan una mayor exploración de las PDLSCs como un enfoque de terapia celular viable para las enfermedades neurodegenerativas que afectan al nervio óptico.
Palabras clave: Células Madre; Células Ganglionares de la Retina (RGC); Células Madre Derivadas del Ligamento Periodontal Humano (PDLSCS); Neuropatías Ópticas; Vesículas Extracelulares (EVS).
INTRODUCTION
Retinal Ganglion Cell (RGC) degeneration is a frequent pathologic condition root of optic neuropathies and glaucoma, which together account for over 100 million individuals globally and are the major factor of permanent blindness and vision loss in industrialized countries. For certain patients, reducing intraocular pressure can halt the course of glaucoma. Additionally, there is currently no cure for visual neuropathies. Furthermore, the human retina has a limited capacity for regeneration, and the degraded RGCs in glaucoma cannot be restored. It is necessary to find novel therapeutic approaches to combat RGC degeneration. The two main approaches to treating glaucoma and optic neuropathy are cell replacement and neuroprotection. RGCs created from stem cells should be an efficient treatment for sick or degraded cells.(1) The outer nuclear layer (ONL) houses the cell bodies of rods and cones, which detect and form synaptic connections with secondary neurons. The Inner Nuclear Layer (INL) contains the cell bodies of retinal interneurons, which include bipolar and amacrine cells. Tissue grafting, laser treatment, and the new authorization of the first gene treatment for RPE-65-associated retinal degeneration are notable advancements in the progress of eye health. The eye is also at the forefront of cell treatment for advanced diseases that involve cell loss, and intriguing to the potential for future cell and gene therapy combinations.(2)
The ongoing pursuit of treatments that enhance RGC existence and axonal renewal has led to numerous lab research. An established model of intra-orbital optic nerve crush causes RGCs to gradually degenerate, primarily through apoptosis. Despite entering a regenerative state following azonal injury marked by the upregulation of advanced related protein 43 and activation of transcript aspect 3, among other aspects, RGCs fail to effectively extend axons beyond the lesion site. The regeneration failure is caused by both intrinsic neuronal factors and elements, which include glial cells and extracellular matrix. Additionally, damage to the optic nerve disrupts the link with axonal targets such as the superior Colliculus (SC) that leads to a disappointment in the transfer of trophic nutrients.(3) A better understanding of glaucomatous neurodegeneration has been made possible by the development of several animal models; however, these models frequently have physiological characteristics that differ from those found in human patients. Additionally, new research showing notable differences between RGCs in rodents and primates raises the possibility that these cells' responses to glaucomatous damage vary significantly between species. To ascertain the pathophysiology of RGCs and the mechanisms underlying the degeneration and death, it is imperative that novel methods be developed to supplement established glaucoma models.(4) To analyze the neuroprotective and regenerative probable of Extracellular Vesicles (EVs) generated from human periodontal ligament-derived stem cells (PDLSCs) in a model of rat ocular neuropathies. The research aims to determine whether PDLSCs can enhance axonal regeneration, prevent apoptosis, and promote RGC survival. The analysis next explores the role of paracrine signaling by comparing the effect of serum-starved PDLSCs and figuring out whether EVs alone can provide the same therapeutic benefits. Figure 1 shows the impacts of Saline injection on retinal function, RGC loss, and inflammation.
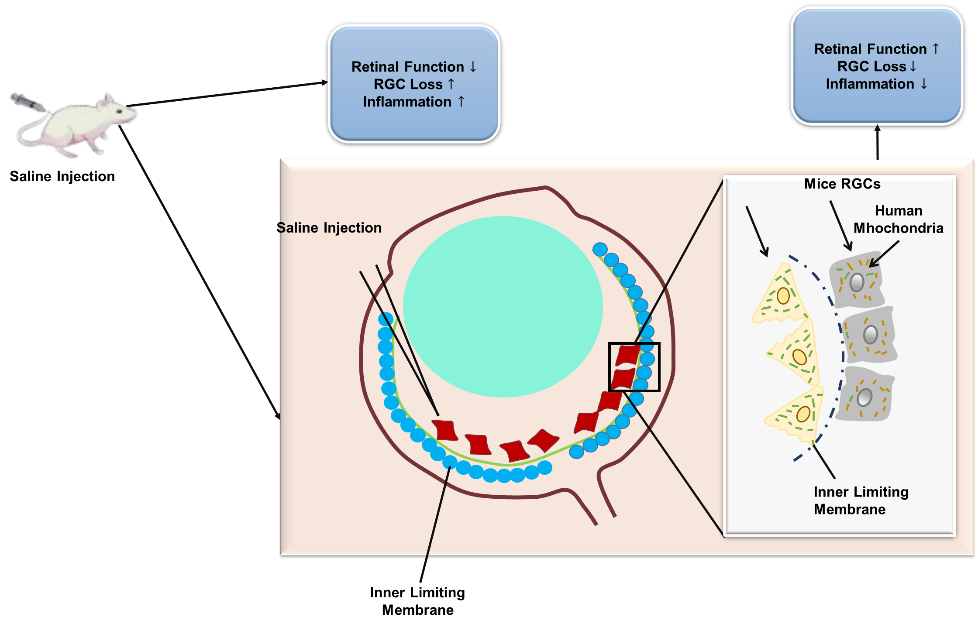
Figure 1. The impacts of Saline injection on retinal function, RGC loss, and inflammation
An indication of the challenges in applying stem cell-based neuroprotective therapies for the replacement and protection of RGCs in the clinic was highlighted.(5) Mesenchymal stem cells (MSCs), cell-free stem cell therapy, and genome editing technologies, like CRISPR-Cas9 and TALEN®, were being explored in the research as potential means to enhanced neuroprotection. Results indicated that MSCs enhanced the survival of RGCs and that genome editing and cell-free therapy can result in clinically compatible RGCs. However, limitations like the inability of MSCs to enter the GCL, the insufficient availability of productive differentiation protocols, and the inability to identify definitive markers that enable RGCs to be differentiated from other neurons. To overcome the challenges and develop accurate and effective treatments for restoring visual function, pharmaceutical, bioengineering, and gene therapy strategies need to be integrated.
The challenges of bringing RGC therapies from the experimental literature to the clinic, with particular emphasis on neuroprotection, gene therapy, and cell replacement must be addressed.(6) Over 100 various therapeutic strategies have been tested in vitro, in animal models, and early clinical trials, ranging from pharmacologic agents to dietary supplements, neurotrophic factors, and viral vector-mediated gene therapy. While cell replacement therapy was limited by integration concerns, the value of restoring full vision justified further research. Neuroprotection has been rewarded, particularly in glaucoma, and gene therapy has also produced hopeful results in genetic disorders like Leber's Hereditary Optic Neuropathy (LHON). Clinical practice translation was indeed prevented by disagreement between animal models and human biology, the constraint in simulating neurodegenerative disease models, and the diversity of therapeutic outcomes. Additionally, encouraging early results have not always been reproduced in randomized controlled trials due to still-unsolved problems such as treatment duration, dose optimization, and patient selection criteria.
The possibility of stem cell-based treatment for the replacement of RGCs was discussed for the treatment of optic neuropathies, primarily glaucoma.(7) Approaches involved analysis of ways to generate, detect, and transplantable RGCs from pluripotent stem cells (PSCs), as well as investigation of the suitability for disease modeling and drug screening. Results presented the promise of RGCs derived from stem cells as opening new doors for visual recovery therapies. Nonetheless, additional improvements were necessary to improve RGC survival, differentiation, and integration with retinal and brain targets, and also to break through the central nervous system's resistance to regeneration to achieve complete functional recovery. Available cell sources and technical application challenges were emphasized while giving an indication of the capability of cell therapies to regenerate or substitute lost and injured cells in retinal degeneration and optic nerve disorders.(8) The methods involved consideration of various types of stem cells, including MSCs, PSCs, and Retinal Progenitor Cells (RPCs), that have progressed into early-stage clinical trials due to the safety profile and ability for differentiation. The cell therapies have the potential to cure retinal diseases and were founded to have some success but without any compromise on safety. However, more effort must be made to counteract hazards such as tumor development and surgical issues with transplantation, enhance cell survival, delivery strategies, enrichment for specific retinal subtypes, and integration. Possible treatments for demyelinating optic neuritis, inflammatory demyelination of the optic nerve linked with multiple sclerosis, and other diseases were explored.(9) Monoclonal antibodies against inflammatory processes and immunosuppressive drugs have been effective in clinical trials. MSC therapy has demonstrated demyelinating and anti-inflammatory effects in small clinical trials. However, it remains difficult to deliver MSC to the retina and optic nerve, necessitating different approaches. Since MSC-derived Extracellular Vesicles (EVs) exhibited neuroprotective activities and possess the capability to traverse the blood-brain barrier, it potentially could be an excellent cell-free therapy. To provide effective clinical implementation, further research must be undertaken to maximize EV efficiency using gene editing, miRNA modification, and hydrogel-based drug delivery systems. The ocular neuropathies, which lead to vision loss through damage to RGCs, the axons, and glia, were the focus.(10) To encourage RGC existence and axonal revival, preclinical research in tiny rats has examined various strategies, including gene deletion, pharmacologic treatments, and visual stimulation. The combination approaches have yielded robust target reconnection and axonal regrowth. In addition, cell replacement strategies and paracrine active cell treatments were under investigation. To enhance regeneration and functional restoration, future research should focus on axon-sparing and glia-sparing therapy for acute and chronic ocular neuropathies. Such treatments could be combined with existing RGC-targeted treatments.
METHOD
Optic neuropathies outcome in permanent vision loss as a consequence of RGC degeneration and axonal damage, limited therapeutic interventions. Stem cell therapies, especially PDLSCs, have demonstrated potential in the induction of neuroprotection and regeneration. In this research, the effectiveness of PDLSC transplantation and EV therapy is assessed in a rat model of optic nerve damage in terms of RGC survival, axonal regeneration, and functional recovery. Apart from that, PDLSCs deprived of serum were examined to investigate the preconditioning effect in raising neuroprotection. The findings are anticipated to provide explanations on whether EV-based therapy can rival the benefits of whole-cell transplantation and validate PDLSCs as an optic neuropathies therapy.
PDLSC Isolation and Characterization
It is required to detect, nurture, and verify PDLSCs for transplantation. The explant culture technique utilized to culture PDLSCs from human premolars by disrupting periodontal ligament tissues and placing them in fibronectin-coated dishes was to stimulate cell migration. The cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) enriched with 10 % platelet lysate and further subculture at 80 % confluence. The multipotency is verified by tri-lineage differentiation tests that also verified the adipogenic, osteogenic, and chondrogenic potential and the suitability for use in therapeutics.
Experimental Design and Animal Model
An optic nerve crush (ONC) model of damage is utilized in rats to assess the beneficial latent of PDLSCs and their extracellular vesicles (EVs) in optic neuropathy. ONC is a well-established method that replicates axonal damage and RGC loss, characteristic of human ocular neuropathies. To evaluate the impacts of different therapies, four experimental groups were created after inducing the damage.
PDLSC Transplantation Group
Live PDLSCs were intravitreally injected into the rats in the group. They attempt to evaluate direct cell-mediated neuroprotection and determine whether PDLSCs can enhance axonal regeneration and RGC survival through both paracrine signaling and cell replacement. PDLSCs are critical to assess the overall therapeutic ability due to the reported capacity to integrate with retinal cells, secrete neurotrophic factors, and regulate inflammation.
EV Transplantation Group
Rats in the group are administered isolated extracellular vesicles (EVs) derived from PDLSCs instead of intact PDLSCs. Bioactive compounds in EVs, such as growth factors, microRNAs, and proteins, have been found to mediate cell-to-cell communication and heal tissues. To possibly reduce the risks of live cell transplantation, the objective is to investigate whether EVs in isolation could potentially provide the same neuroprotective and regenerative benefits as PDLSC implantation in an EV cell-free format.
Serum-Deprived PDLSC Group
PDLSCs is preconditioned in serum-free conditions before transplantation were administered to the group.The process of serum deprivation induces stress and has been shown to encourage stem cells to release neurotrophic factors and other beneficial signaling molecules. Preconditioned PDLSCs were tested to see if it could improve RGC survival and axonal regeneration, and if it had more neuroprotective benefits than regular PDLSCs.
Control Group
The control group for rats received no PDLSCs or EVs; it received a saline injection instead. The group is used to determine the degree to which the PDLSC and EV treatments added to outcomes beyond normal healing mechanisms.
Histological and behavioral analyses were used to evaluate RGC survival rates, axonal regeneration, and the functional recovery of visual pathways at 15, 61, and 120 days after the lesion.
Transplantation Procedure
PDLSCs and EVs ensure that they are accurately delivered into the vitreous chamber. PDLSCs were cultured in sterile conditions before transplantation and are analyzed by fluorescence-activated cell sorting (FACS). EVs were isolated from a PDLSC-conditioned medium by ultracentrifugation (100 000 × g for 90 minutes at 4°C), and size distribution is verified by nanoparticle tracking analysis. A Hamilton syringe (33G needle) inserted under a surgical microscope to administer each treatment ensures accurate intravitreal injection without damaging the retina or lens. Rats were monitored post-treatment to ensure recovery and acted normally.
RESULTS
EVs, serum-free PDLSCs, and PDLSC transplantation were assessed for the regenerative and neuroprotective activity on RGC survival, apoptosis inhibition, axonal regeneration, and practical restoration in a rat model of optic neuropathy. The results indicate that PDLSC implantation, particularly 120 days post-damage, significantly enhanced axonal regeneration, reduced apoptosis, and enhanced RGC survival. Although EV transplantation alone could not induce significant neuroprotection or axonal regeneration, serum-deprived PDLSCs also exhibited similar neuroprotective activities, indicating the possibility of paracrine signaling.
Histological and immunofluorescence Analysis
Pentobarbital overdose (100 mg/kg) employ to euthanize rats at15, 61, and 120 days after injury, and the eyes were enucleated for processing of the retina and optic nerve. Samples of retinal and optic nerve tissue were fixed in 4 % paraformaldehyde (PFA), cryoprotected with 30 % sucrose, and then sectioned using a cryostat at a width of 10 μm. Sections were immunostained by nurturing the primary antibodies to the RGC marker βIII-tubulin, the axonal regeneration marker GAP43, and the apoptotic marker Caspase-3. Secondary antibodies conjugated with Alexa Fluor were then applied.
Microscopy: A Zeiss LSM 800 confocal microscope is employed to capture fluorescent images, and RGC survival is quantified in randomized fields throughout retinal sections
.
Retinal Ganglion Cell (RGC) Survival and Apoptosis
RGC survival is assessed through βIII-tubulin immunostaining at 15, 61 and 120 days following damage. RGC survival rates were markedly higher in PDLSC-treated animals associated with control and EV-treated animals; the most significant alterations were observed at 15 and 61 days. The survival rates of serum-deprived PDLSCs were comparable, showing that preconditioning enhances the viability of therapeutic application. With Caspase-3 staining to quantify apoptosis levels, the PDLSC-treated groups were found to have significantly fewer apoptotic RGCs compared to controls. The PDLSC group has a decrease in apoptosis of approximately 60 % at 15 days, and this effect persisted at 120 days, showing long-term neuroprotective benefits. EV transplantation did not, however, significantly reduce apoptosis, indicating that direct cellular interaction may be necessary for long-term neuroprotection. Table 1 and figure 2 represent the RGC survival and apoptosis cell treatment groups.
|
Table 1. RGC Survival and Apoptosis Reduction |
||||
|
Parameter |
Control |
EV Transplantation |
PDLSC Transplantation |
Serum-Deprived PDLSCs |
|
RGC Survival |
40,5 ± 3,2 |
45,8 ± 3,7 |
40,5 ± 3,2 |
76,9 ± 4,5 |
|
Apoptotic Cells (Caspase-3, positive) |
55,1 ± 4,5 |
50,7 ± 3,9 |
22,8 ± 2,7 |
24,3 ± 2,9 |
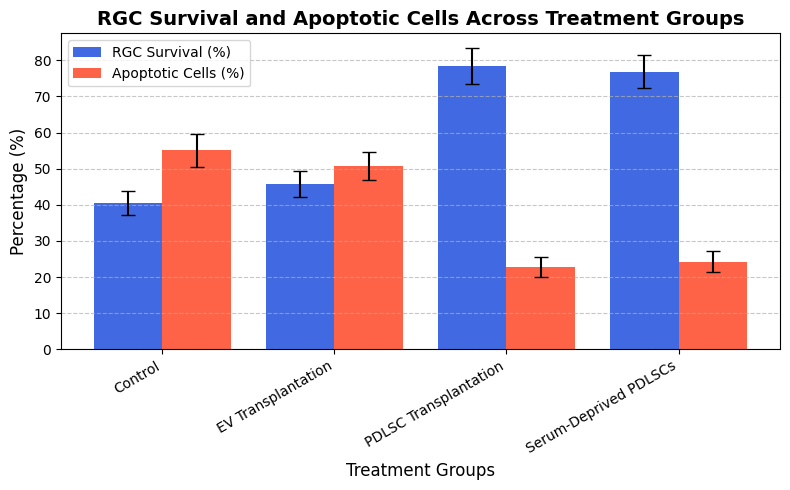
Figure 2. RGC Survival and Apoptotic Cell Treatment Groups
The control group's RGC survival rate is 40,5 ± 3,2, the EV transplant group's is 45,8 ± 3,7, the PDLSC transplant group's is 78,4 ± 4,9, and the serum-deprived PDLSC group's is 76,9 ± 4,5. The control group had 55,1 ± 4,5 percent apoptotic cells (Caspase-3 positive), the EV transplant group had 50,7 ± 3,9, the PDLSC transplant group had 22,8 ± 2,7, and the serum-deprived PDLSC group had 24,3 ± 2,9.
Axonal regeneration quantification
To identify restoring axons in the optic nerve, Cholera Toxin B (CTB-488, 1 μL, administered intravitreally 7 days before death) is used. Quantification is done on the count of GAP43-expressing axons that extended 250 μm and 500 μmbeyond the injury site. Equation (1) is used to determine axonal density.
![]()
Where the overall count of regenerating axons at distance d is denoted by R(d). The optic nerve radius is denoted by πr, and the thickness of the segment is denoted by t.
GAP43 immunostaining is performed to evaluate axonal regeneration 120 days following the lesion. Axons extending up to 500 µm from the site of the lesion exhibited extensive axonal regrowth after PDLSC implantation. The same patterns of regeneration were exhibited by serum-deprived PDLSCs, validating the role of paracrine signaling. Nevertheless, no extensive axonal extension is achieved through EV transplantation, which points towards the necessity of cellular contact for regeneration. Table 2 and figure 3 show the axonal regeneration at 120 days post-injury.
|
Table 2. Axonal Regeneration at 120 days Post-Injury |
||||
|
Distance from Injury Site |
Control |
EV Transplantation |
PDLSC Transplantation |
Serum-Deprived PDLSCs |
|
250 μm |
15,2 ± 3,1 |
22,7 ± 3,9 |
68,5 ± 4,7 |
65,2 ± 4,9 |
|
500 μm |
6,8 ± 2,5 |
12,4 ± 3,2 |
49,8 ± 5,2 |
46,3 ± 5,0 |
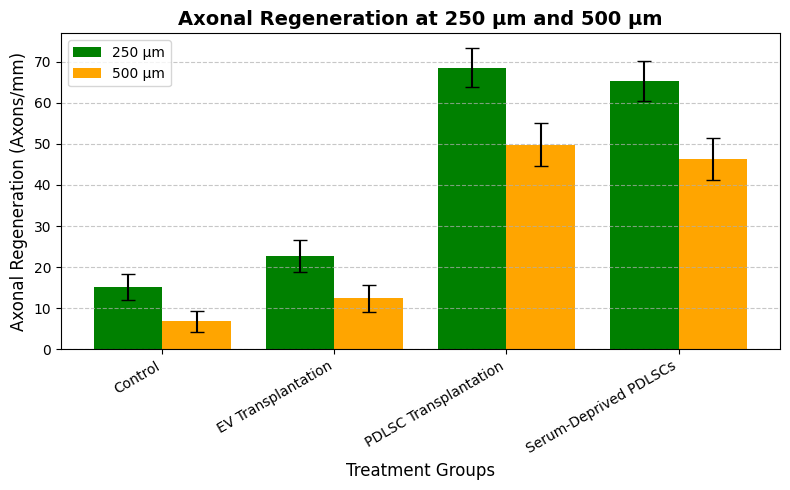
Figure 3. Axonal Regeneration at 250 µm and 500 µm
The axonal regeneration at 250 μm from the injury site is 15,2 ± 3,1 in the control class, 22,7 ± 3,9 in the EV transplantation class, 68,5 ± 4,7 in the PDLSC transplantation class, and 65,2 ± 4,9 in the serum-deprived PDLSC class. At 500 μm, the axonal regeneration is 6,8 ± 2,5 in the control class, 12,4 ± 3,2 in the EV transplantation class, 49,8 ± 5,2 in the PDLSC transplantation group, and 46,3 ± 5,0 in the serum-deprived PDLSC group.
Gene Expression analysis
The TRIzol method is employed to isolate total RNA from retinal tissues at 15, 61, and 120 days following the damage. Reverse transcription is employed to create cDNA, and qRT-PCR is employed to investigate the presence of retinal progenitor creators, RGC markers, and neurotrophic factors (CNTF,GDNF, and BDNF). Relative fold changes were planned using the 2(-ΔΔCt) method following the normalization of gene expression data against Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Table 3 and figure 4 display the gene expression across treatment classes.
|
Table 3. Relative Fold Change in Neurotropic Gene Expression |
||||
|
Gene |
Control |
EV Transplantation |
PDLSC Transplantation |
Serum-Deprived PDLSCs |
|
BDNF |
1,0± 0,1 |
1,3± 0,2 |
4,8± 0,5 |
4,5±0,4 |
|
CNTF |
1,0± 0,1 |
1,5± 0,3 |
5,2± 0,6 |
4,9±0,5 |
|
GDNF |
1,0± 0,2 |
1,4 ± 0,3 |
3,9± 0,4 |
3,7±0,3 |
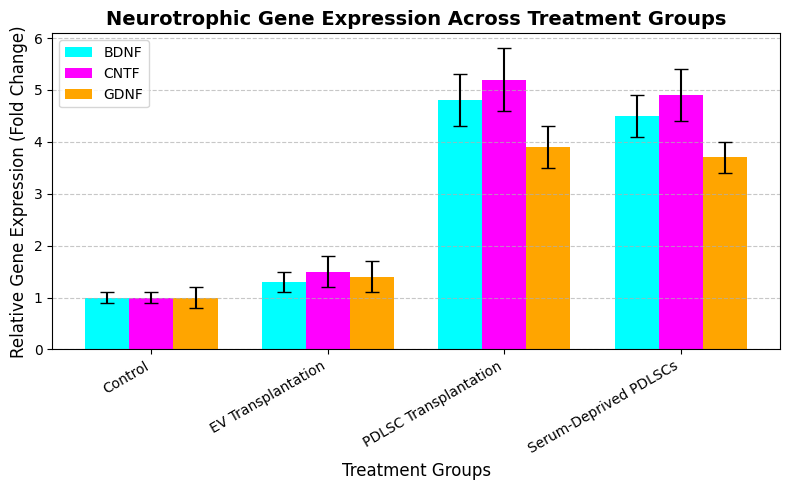
Figure 4. Neurotrophic Gene Expression Across Treatment Groups
The BDNF expression is 1,0 ± 0,1 in the control class, 1,3 ± 0,2 in the EV transplantation class, 4,8 ± 0,5 in the PDLSC transplantation class, and 4,5 ± 0,4 in the serum-deprived PDLSC class. The CNTF expression is 1,0 ± 0,1 in the control class, 1,5 ± 0,3 in the EV transplantation class, 5,2 ± 0,6 in the PDLSC transplantation class, and 4,9 ± 0,5 in the serum-deprived PDLSC class. The GDNF expression is 1,0 ± 0,2 in the control class, 1,4 ± 0,3 in the EV transplantation class, 3,9 ± 0,4 in the PDLSC transplantation class, and 3,7 ± 0,3 in the serum-deprived PDLSC class.
Functional recovery assessment
The following methods are used to evaluate visual function.
The optomotor response test measures visual acuity by analyzing spatial frequency thresholds.
RGC activity is measured in electrophysiology (Pattern Electroretinography, PERG) to assess functional recovery. Table 4 and figure 5 express the optomotor response and PERG amplitude across treatment classes.
|
Table 4. Functional Recovery at 15,61, and 120 days Post-Injury |
||||
|
Parameter |
Control |
EV Transplantation |
PDLSC Transplantation |
Serum-Deprived PDLSCs |
|
Optomotor Response |
1,3 ± 0,2 |
1,6 ± 0,3 |
3,7 ± 0,4 |
3,4 ± 0,3 |
|
PERG Amplitude (μV) |
1,3 ± 0,2 |
2,6 ± 0,4 |
6,8 ± 0,5 |
6,3 ± 0,4 |
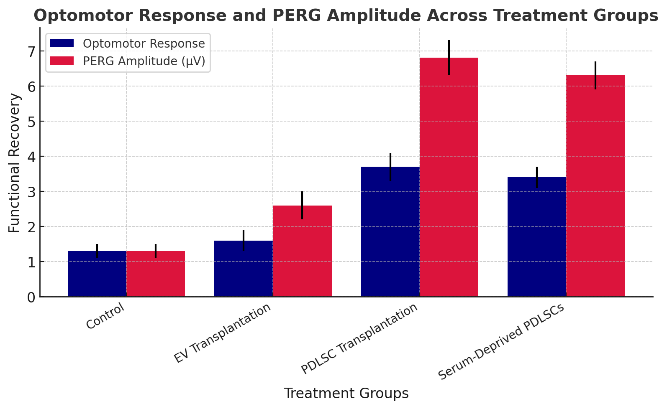
Figure 5. Optomotor Response and PERG Amplitude Across Treatment Groups
The optomotor response is 1,3 ± 0,2 in the control class, 1,6 ± 0,3 in the EV transplantation class, 3,7 ± 0,4 in the PDLSC transplantation class, and 3,4 ± 0,3 in the serum-deprived PDLSC class. The PERG amplitude (µV) is 1,3 ± 0,2 in the control class, 2,6 ± 0,4 in the EV transplantation class, 6,8 ± 0,5 in the PDLSC transplantation group, and 6,3 ± 0,4 in the serum-deprived PDLSC group.
DISCUSSION
The therapeutic capability of PDLSCs and EVs in RGC survival, axonal renewal, and practical retrieval in a rat model of optic neuropathy was evaluated. Current treatment strategies, including neurotrophic factor therapy, gene therapy, and pharmacological interventions, provide limited success in restoring lost RGCs and regenerating axons. Although stem cell-based methods hold potential, limitations in the form of low survival, immune rejection, and variable therapeutic responses exist. EV-based therapy has been analyzed as a cell-free alternative but has limitations due to low retention of bioactive molecules and a lack of direct cell-cell interaction. This research augments the ability to protect retinal cells and promote long-term survival following injury. To restore the connectivity of the visual pathway, it facilitates the regeneration of the damaged nerve fibers. The analysis also demonstrates that preconditioning of the cells before transplantation increases the effectiveness by promoting more release of beneficial factors. By re-establishing responsiveness and communication between brain and retinal cells, this operation, as opposed to other operations, restores vision with striking effect. It also unlocks the processes at the molecular level that underlie recovery and underscores the role that particular proteins play in the restoration of nerve. These findings suggest that PDLSC transplantation is a promising strategy for optic neuropathies.
CONCLUSIONS
The research is to assess the degree to which EV treatment and PDLSC transplantation can enhance RGC survival, axonal regeneration, and functional recovery in a rat optic neuropathy model. Serum deprivation is also explored as a preconditioning method to enhance the neuroprotective effects of PDLSCs. PDLSCs were isolated, characterized, and intravitreally implanted, while EVs were administered alone to evaluate the efficacy independently. For evaluating spontaneous healing, saline injections were administered to a control group. At 15, 61, and 120 days after optic nerve crush (ONC) injury induction, histological, functional, and molecular investigations were performed. With evident benefits at 15 and 61 days and sustained benefits up to 120 days, the findings revealed that implantation of PDLSC significantly enhanced RGC survival and reduced apoptosis. Axonal regeneration is the best in groups treated with PDLSC, but EV transplantation alone could not significantly restore the damaged areas, which suggests that direct cellular contact is required for optimal regeneration. The preconditioning serum-deprived PDLSCs exhibited neuroprotective effects, emphasizing the importance of preconditioning. Gene expression analysis confirmed the up regulation of critical neurogenic factors (CNTF, GDNF, and BDNF,), and functional recovery tests also support the improved performance of PDLSC-treated groups. EV therapy must be more optimized, and the long-term survival of PDLSCs after transplantation is unknown even though results are encouraging. For an assessment of clinical use in ocular neuropathies and neurodegenerative disease, further research should target enhanced EV bioactivity, explorations of biomaterial scaffolds for PDLSCs, and preclinical models in larger animal models.
BIBLIOGRAPHIC REFERENCES
1. Cen LP, Ng TK. Stem cell therapy for retinal ganglion cell degeneration. Neural Regeneration Research. 2018 Aug 1;13(8):1352-3. https://doi.org/10.4103/1673-5374.235237
2. Boia R, Ruzafa N, Aires ID, Pereiro X, Ambrósio AF, Vecino E, Santiago AR. Neuroprotective strategies for retinal ganglion cell degeneration: current status and challenges ahead. International journal of molecular sciences. 2020 Mar 25;21(7):2262. https://doi.org/10.3390/ijms21072262
3. da Silva-Junior AJ, Mesentier-Louro LA, Nascimento-dos-Santos G, Teixeira-Pinheiro LC, Vasques JF, Chimeli-Ormonde L, Bodart-Santos V, de Carvalho LR, Santiago MF, Mendez-Otero R. Human mesenchymal stem cell therapy promotes retinal ganglion cell survival and target reconnection after optic nerve crush in adult rats. Stem Cell Research & Therapy. 2021 Dec;12:1-4. https://doi.org/10.1186/s13287-020-02130-7
4. VanderWall KB, Huang KC, Pan Y, Lavekar SS, Fligor CM, Allsop AR, Lentsch KA, Dang P, Zhang C, Tseng HC, Cummins TR. Retinal ganglion cells with a glaucoma OPTN (E50K) mutation exhibit neurodegenerative phenotypes when derived from three-dimensional retinal organoids. Stem Cell Reports. 2020 Jul 14;15(1):52-66. https://doi.org/10.1016/j.stemcr.2020.05.009
5. Behtaj S, Rybachuk M. Strategies on the application of stem cells based therapies for the treatment of optic neuropathies. Neural Regeneration Research. 2021 Jun 1;16(6):1190-1. https://orcid.org/0000-0001-6589-3119
6. Fu L, Kwok SS, Chan YK, Ming Lai JS, Pan W, Nie L, Shih KC. Therapeutic strategies for attenuation of retinal ganglion cell injury in optic neuropathies: concepts in translational research and therapeutic implications. BioMed research international. 2019;2019(1):8397521. https://doi.org/10.1155/2019/8397521
7. Rabesandratana O, Goureau O, Orieux G. Pluripotent stem cell-based approaches to explore and treat optic neuropathies. Frontiers in neuroscience. 2018 Sep 20;12:651 https://doi.org/10.3389/fnins.2018.00651
8. Coco-Martin RM, Pastor-Idoate S, Pastor JC. Cell replacement therapy for retinal and optic nerve diseases: cell sources, clinical trials and challenges. Pharmaceutics. 2021 Jun 11;13(6):865. https://doi.org/10.3390/pharmaceutics13060865
9. Aneesh A, Liu A, Moss HE, Feinstein D, Ravindran S, Mathew B, Roth S. Emerging concepts in the treatment of optic neuritis: mesenchymal stem cell-derived extracellular vesicles. Stem Cell Research & Therapy. 2021 Dec;12:1-5. https://doi.org/10.1186/s13287-021-02645-7
10. Mesentier-Louro LA, Liao YJ. Optic nerve regeneration: considerations on treatment of acute optic neuropathy and end-stage disease. Current Ophthalmology Reports. 2019 Mar 15;7:11-20. https://doi.org/10.1007/s40135-019-00194-0
FINANCING
None.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Jamuna KV, Uma Bhardwaj, Surjya Prakash S.
Data curation: Jamuna KV, Uma Bhardwaj, Surjya Prakash S.
Formal analysis: Jamuna KV, Uma Bhardwaj, Surjya Prakash S.
Drafting - original draft: Jamuna KV, Uma Bhardwaj, Surjya Prakash S.
Writing - proofreading and editing: Jamuna KV, Uma Bhardwaj, Surjya Prakash S.