doi: 10.56294/mw2024517
ORIGINAL
AI-Powered Knowledge Graphs for Efficient Medical Information Retrieval and Decision Support
Gráficos de conocimiento basados en IA para una recuperación eficiente de información médica y apoyo a la toma de decisiones
Santanu
Kumar Sahoo1 ![]() *, Manni Sruthi2
*, Manni Sruthi2 ![]() , Varun Ojha3
, Varun Ojha3 ![]() , Vaibhav Kaushik4
, Vaibhav Kaushik4 ![]() , Manti Debnath5
, Manti Debnath5 ![]() , Renuka Jyothi.S6
, Renuka Jyothi.S6 ![]() , Naresh Kaushik7
, Naresh Kaushik7 ![]()
1Department of Electronics and Communication Engineering, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India.
2Centre for Multidisciplinary Research, Anurag University, Hyderabad, Telangana, India.
3Chitkara Centre for Research and Development, Chitkara University, Himachal Pradesh, India.
4Centre of Research Impact and Outcome, Chitkara University, Rajpura, Punjab, India.
5School of Allied Health Sciences, Noida International University, Greater Noida, Uttar Pradesh, India.
6Biotechnology and Genetics, JAIN (Deemed-to-be University), Bangalore, Karnataka, India.
7Department of uGDX, ATLAS SkillTech University, Mumbai, Maharashtra, India.
Cite as: Kumar Sahoo S, Sruthi M, Ojha V, Kaushik V, Debnath M, S R, et al. AI-Powered Knowledge Graphs for Efficient Medical Information Retrieval and Decision Support. Seminars in Medical Writing and Education. 2024; 3:517. https://doi.org/10.56294/mw2024517
Submitted: 28-10-2023 Revised: 11-02-2024 Accepted: 13-06-2024 Published: 14-06-2024
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding author: Santanu Kumar Sahoo *
ABSTRACT
The enormous volume of medical data has resulted in the development of sophisticated systems that facilitate information search and enable clinicians in decision-making process. Driven by artificial intelligence, knowledge graphs (KGs) provide a solid structure for organising and evaluating vast volumes of diverse medical data, therefore enabling wiser question development and improved decision-making. This article presents a whole strategy for integrating knowledge graphs with artificial intelligence-based approaches to improve medical information search and decision support systems performance. Graph-based reasoning, natural language processing (NLP), and machine learning all help the proposed approach to enhance semantic comprehension. It achieves this by tying together unorganised and organised medical data sources to provide pertinent analysis. Using predictive analytics, personalised healthcare recommendations, and real-time clinical decision support, the AI-powered knowledge graph architecture helps you It achieves this by continuously shifting the relationships among illnesses, symptoms, therapies, pasts of patients. This approach also ensures that many healthcare systems may cooperate better, which facilitates information search and reduces the diagnostic error count. Including reinforcement learning techniques enhances question results depending on user interaction, therefore enhancing the search process. The results of experiments show that KGs with AI work better than traditional database-driven methods when it comes to getting medical information quickly, correctly, and usefully. The suggested method helps healthcare workers a lot by making it easier for them to get accurate, evidence-based information more quickly. This will eventually lead to better patient results. This study shows that knowledge graphs driven by AI have the ability to completely change how medical information is managed and how decisions are made. This could lead to smarter and more flexible healthcare systems.
Keywords: AI-Powered Knowledge Graphs; Medical Information Retrieval; Clinical Decision Support; Natural Language Processing; Healthcare Interoperability.
RESUMEN
El enorme volumen de datos médicos ha dado lugar al desarrollo de sofisticados sistemas que facilitan la búsqueda de información y permiten a los clínicos tomar decisiones. Impulsados por la inteligencia artificial, los grafos de conocimiento (KG) proporcionan una estructura sólida para organizar y evaluar grandes volúmenes de datos médicos diversos, lo que permite elaborar preguntas más sabias y mejorar la toma de decisiones. Este artículo presenta toda una estrategia para integrar los grafos de conocimiento con planteamientos basados en la inteligencia artificial a fin de mejorar la búsqueda de información médica y el rendimiento de los sistemas de apoyo a la toma de decisiones. El razonamiento basado en grafos, el procesamiento del lenguaje natural (PLN) y el aprendizaje automático ayudan al planteamiento propuesto a mejorar la comprensión semántica.Esto se consigue uniendo fuentes de datos médicos organizadas y no organizadas para proporcionar análisis pertinentes. Mediante el análisis predictivo, las recomendaciones sanitarias personalizadas y el apoyo a la toma de decisiones clínicas en tiempo real, la arquitectura de grafos de conocimiento impulsada por IA le ayuda a Consigue esto desplazando continuamente las relaciones entre enfermedades, síntomas, terapias y pasados de los pacientes. Este enfoque también garantiza que muchos sistemas sanitarios puedan cooperar mejor, lo que facilita la búsqueda de información y reduce el número de errores de diagnóstico. La inclusión de técnicas de aprendizaje por refuerzo mejora los resultados de las preguntas en función de la interacción del usuario, lo que mejora el proceso de búsqueda. Los resultados de los experimentos demuestran que las KG con IA funcionan mejor que los métodos tradicionales basados en bases de datos a la hora de obtener información médica de forma rápida, correcta y útil. El método sugerido ayuda mucho al personal sanitario al facilitarle la obtención de información precisa y basada en pruebas con mayor rapidez. Con el tiempo, esto se traducirá en mejores resultados para los pacientes. Este estudio demuestra que los gráficos de conocimiento impulsados por la IA tienen la capacidad de cambiar por completo la forma en que se gestiona la información médica y se toman las decisiones. Esto podría dar lugar a sistemas sanitarios más inteligentes y flexibles.
Palabras clave: Grafos de Conocimiento Impulsados por IA; Recuperación de Información Médica; Apoyo a la Toma de Decisiones Clínicas; Procesamiento del Lenguaje Natural; Interoperabilidad Sanitaria.
INTRODUCTION
Medical knowledge has soared thanks in great part to biomedical research, electronic health records (EHRs), and clinical investigations. This has produced a vast volume of both ordered and unorganised medical records. To make wise decisions, improve patient outcomes, and expedite clinical procedures, healthcare professionals must be able to efficiently locate and use this massive information bank. Conventional database-driven approaches for obtaining medical information, however, can have issues such not understanding the context, being sluggish when handling various kinds of data, and difficulties aggregating data from many sources.(1) Driven by artificial intelligence, knowledge graphs (KGs) have become a promising approach to address these issues as they provide an ordered, scalable, semantically rich means to manage medical data. Strong data structures, knowledge graphs demonstrate how intricately linked in medicine, including illnesses, symptoms, therapies, drugs, and patient records, are many elements. Using artificial intelligence (AI), particularly natural language processing (NLP), machine learning (ML), and logic tools, knowledge graphs may help individuals locate information more quickly, properly, and in context. Unlike conventional relational databases that employ fixed architecture and query patterns, AI-enhanced KGs dynamically mix several data sources, extract important insights, and generate smart recommendations based on evolving medical knowledge.(2) By allowing clinicians to immediately get tailored, valuable medical information, the capacity to do this greatly improves clinical decision support systems (CDSS). Combining knowledge graphs with artificial intelligence driven techniques offers various transforming results. First, NLP techniques let medical concepts to be automatically extracted from clinical texts, patient data, and research files and connected.(3) This facilitates finding your way across challenging medical information. Second, machine learning models improve the ability of knowledge graphs to predict the future. This lets us find trends in how diseases get worse, figure out how well treatments work, and divide patients into groups based on their risk. Third, thinking algorithms help people make better decisions by finding hidden connections between medical concepts. This way, healthcare workers can get more information than just what they know. Also, reinforcement learning can be used to improve the process of getting information by making search terms better based on how users interact with them. This makes tips more accurate and useful over time.(4,5)
One big benefit of KGs driven by AI is that they can make it easier for different healthcare systems to work together. At the moment, medical information is spread out among many different hospitals, study centres, and pharmaceutical systems that store different types of data in different forms. Knowledge graphs make semantic sharing possible by standardising medical terms and connecting different datasets.(6) This brings medical information management closer together and more coherently. This reduces needless effort, enhances medical research team performance, and reduces the possibility of error diagnosis-wise. Though they offer great potential, artificial intelligence-powered knowledge graphs are difficult to build and use in the healthcare sector. Ensuring that the data is accurate, complete, and of good quality is still highly crucial as errors in medical data could lead to erroneous findings and misleading recommendations.(7) Following healthcare guidelines and keeping private patient information secure depend on handling privacy and security issues as well. Examining the ethical questions raised by AI-driven decision assistance is also crucial in order to foster confidence between medical practitioners and patients. These include lessening of prejudice and ensuring that choices may be justified.
Knowledge graphs in healthcare
Components: Nodes, Edges, and Relationships
There are three main parts to a knowledge graph: nodes, lines, and connections. Diseases (like diabetes), symptoms (like tiredness), treatments (like insulin therapy), and drugs (like metformin) are all shown by nodes. These nodes are joined to each other by edges, which show how different medical ideas are linked, such as “causes,” “treated with,” or “associated with.” Relationships in knowledge graphs help people understand context better, which makes AI-driven thinking possible.(8) For example, if a patient has more than one sign, a KG can figure out what disease it might be by looking at similar links in other clinical cases. Because they organise medical information in this way, knowledge graphs make data analysis easier to understand and smarter than with regular relational databases.(9)
Ontologies and Semantic Representations in Medical Knowledge Graphs
Ontologies are very important for organising and standardising knowledge graphs, which makes sure that medical ideas are always related and described in the same way. Medical ontologies, like the Gene Ontology, SNOMED CT, and the Unified Medical Language System (UMLS), help put medical words and connections into groups based on their meaning. By connecting terms that are used in different datasets, these definitions help AI models understand medical data more accurately. With semantic representations, healthcare workers can explore medical records more quickly by using normal language searches instead of strict SQL orders.(10) Knowledge graphs improve connectivity between different healthcare systems by using ontology-driven semantic models. This makes it easier to combine electronic health records (EHRs), scientific research, and clinical study data.
Comparison with Traditional Database Approaches
Traditional relational databases, like SQL-based ones, store health data in tables that are organised and have models that have already been set up. These databases are great at transaction processing and retrieving organised data, but they have trouble with unstructured and semi-structured data, which is common in healthcare and includes things like clinical notes, imaging reports, and DNA sequences. Also, relational databases need complicated joins to get back data that is linked to other data, which can be slow and expensive to run on a large scale for medical purposes.(11) Knowledge graphs, on the other hand, are more adaptable and scalable because they show data as a network of connections instead of set charts. As a result, queries can be run faster, thinking is better, and new information can be gathered from established connections.(12) Also, KGs with AI can automatically add and update new data. This makes them perfect for uses where medical knowledge is always changing, like finding new drugs and personalised medicine. This table 1 highlights the advantages of knowledge graphs over traditional database approaches in handling medical information retrieval and decision support.
|
Table 1. Comparison of Knowledge Graphs and Traditional Database Approaches in Healthcare |
||
|
Parameter |
Knowledge Graphs (KGs) |
Traditional Databases (SQL-Based Systems) |
|
Data Structure |
Graph-based with nodes (entities) and edges (relationships) |
Relational tables with predefined schemas and strict data structures |
|
Handling of Relationships |
Efficiently represents complex relationships between medical entities |
Requires multiple table joins, leading to computational inefficiency |
|
Scalability |
Easily scalable with dynamic updates and integration of new data |
Limited scalability due to rigid schemas and high maintenance costs |
|
Query Flexibility |
Supports semantic and natural language queries using AI and ontologies |
Requires predefined SQL queries with strict syntax and structure |
|
Interoperability |
Integrates various medical ontologies (UMLS, SNOMED CT) for standardized data representation |
Limited interoperability; different databases may require complex integration |
|
Reasoning & Inference |
AI-driven reasoning to infer new insights from existing relationships |
Lacks reasoning capabilities; requires predefined rules and queries |
Ai techniques for enhancing knowledge graphs
In figure 1, demonstrate the system design shows how medical data moves from data sources to decision support apps. The building is made up of several important parts: EHRs, study papers, scientific books, and hospital records are at the top of the data sources package. These sources of data add to the collected data, which is what knowledge extraction is based on.
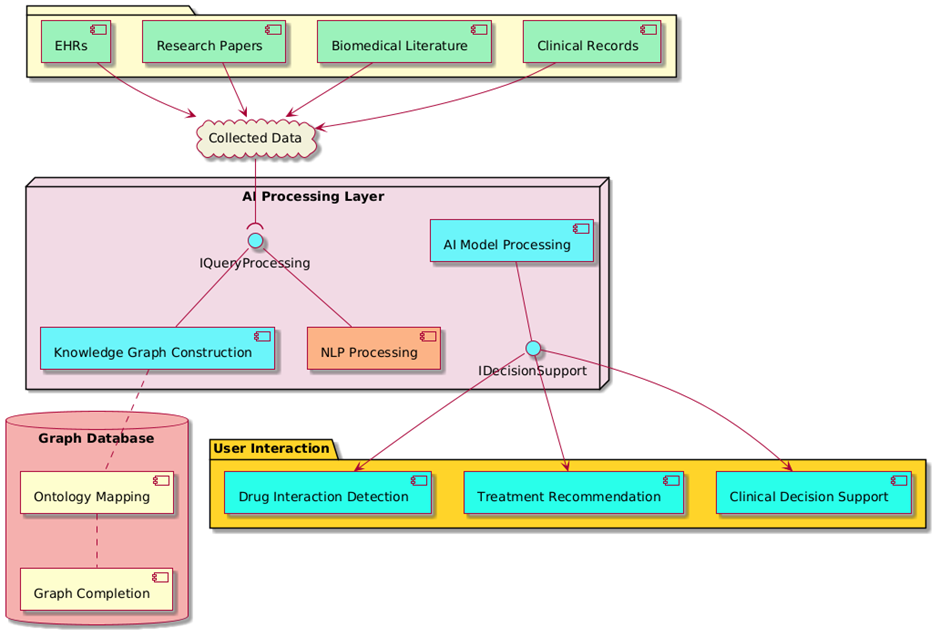
Figure 1. AI-Powered Knowledge Graph architecture for Medical Information Retrieval and Decision Support
The system’s brain is the AI Processing Layer, which is made up of NLP Processing, Knowledge Graph Construction, and AI Model Processing. Using AI methods, these parts work together to find important things, set up connections, and improve knowledge graphs. The IQueryProcessing interface connects the processing layer to the receiving data.
Natural Language Processing (NLP)
Processing large amounts of medical books and patient records to find important ideas, connections, and trends is what information extraction from clinical texts and study papers is all about. Thanks to sophisticated NLP techniques such entity identification, text categorisation, and document summarising, knowledge graphs may mix the most recent research findings with actual patient data. Doctors find it simpler this way while deciding. By identifying medical items in text data like illnesses, symptoms, medications, and treatments named entity recognition (NER) and connection extraction improve knowledge graphs even more. Correct name these objects taught by NER models on medical ontologies such as UMLS and SNOMED CT scan; relationship extraction techniques may establish sensible ties between them. This methodical approach of presenting medical concepts allows knowledge graphs driven by artificial intelligence to easily locate certain information. Based on prior data and clinical evidence, this helps clinicians in diagnosis, treatment recommendations, and illness worsening prediction.
Deep Learning
Deep learning techniques simplify the understanding, organisation, and conclusions drawing process for AI-powered knowledge graphs about medical information from many sources of data. Transformers-based models such BERT and BioBERT are used in entity linking and contextual embeddings to link medical entities with their meanings. This guarantees appropriate understanding of ambiguous terminology, such as “cold” as a symptom against an outside cause. Contextual embeddings let knowledge graphs identify the subtleties of medical terminology and linkages, hence providing accurate information for decision support systems. GNNs for knowledge graph completion estimate where missing linkages exist between medical things, therefore enhancing the AI-powered knowledge graphs for dependability. GNNs use the structure of a knowledge graph to estimate potential connections between nodes, hence enhancing the accuracy and fullness of the graph. Finding hidden connections between symptoms, comorbidities, and treatment efficacy helps identify illnesses early on in clinical decision support, where this is particularly beneficial. Knowledge graphs become flexible and may learn from fresh medical data constantly by employing GNNs. This makes them valuable in fields of medicine undergoing rapid transformation.
Reasoning and Query Optimization
Reinforcement learning systems learn from what doctors say and modify the search words to match, therefore improving their ability to respond to enquiries over time. This guarantees that knowledge graphs provide more precise and practical medical information, thus enabling physicians and nurses to locate correct data for patient diagnosis, treatment planning, and new research design. When reinforcement learning is included, AI-powered knowledge graphs become smart medical assistants that enable individuals to make evidence-based choices and generate individualised healthcare plans.
Applications in medical information retrieval and decision support
Data flow and interoperability
One of the main issues in healthcare is the distribution of medical data across many various organisations, systems, and kinds among many others. AI knowledge graphs generated by standardising medical words across systems tackle this issue. This smooth’s out interoperability and discussion. Sharing patient data may be challenging as various healthcare institutions employ different coding systems—like ICD (International Classification of Diseases), SNOMED CT (Systematised Nomenclature of Medicine), and LOINC (Logical Observation Identifiers Names and Codes). Knowledge graphs combine these many collections of words into one framework. This lets healthcare professionals access correct, standardised medical data across multiple platforms. This makes it easier for hospitals, study centres, and public health bodies to work together, which makes the sharing of medical data more efficient overall. Connecting electronic health records (EHRs) with basic research is another important use. This closes the gap between clinical practice and scientific finding. EHRs have useful information about patients, but their full potential isn’t always used because they store information in separate areas and don’t connect to study libraries. Knowledge graphs backed by AI link electronic health records (EHRs) to medical books, clinical study data, and genetic research. This lets doctors and academics make decisions based on data. This combination makes it possible to match people in clinical trials in real time, find possible candidates for new treatments, and speed up the drug development process.
|
Table 2. Comparison of AI-Powered Knowledge Graph Applications in Medical Information Retrieval and Decision Support |
|||
|
Parameter |
Clinical Decision Support Systems (CDSS) |
Medical Knowledge Discovery |
Interoperability and Data Integration |
|
Primary Function |
Enhancing clinical decision-making through AI-driven insights |
Discovering novel biomarkers, treatment pathways, and disease patterns |
Standardizing medical terminologies and linking heterogeneous datasets |
|
Key Data Sources |
Electronic Health Records (EHRs), patient symptoms, medical guidelines |
Genomic data, clinical trials, biomedical literature, omics datasets |
EHR systems, public health databases, medical terminologies (ICD, SNOMED CT) |
|
Main AI Techniques Used |
Natural Language Processing (NLP), Machine Learning (ML), Reasoning |
Graph Neural Networks (GNNs), Deep Learning, Entity Linking |
Ontology-based AI, Semantic Integration, Knowledge Graph Completion |
|
Impact on Healthcare |
Improves diagnostic accuracy and personalized treatment recommendations |
Accelerates drug discovery, precision medicine, and disease prediction |
Enhances data interoperability, enabling seamless knowledge sharing |
|
Challenges |
Requires high-quality patient data, risk of AI bias, regulatory constraints |
Data heterogeneity, need for extensive validation, interpretability issues |
Data standardization across different institutions, security, and privacy concerns |
|
Example Use Cases |
Differential diagnosis, treatment planning, drug-drug interaction alerts |
Predicting disease progression, identifying molecular biomarkers |
Integrating EHRs with research databases for real-time insights |
|
Scalability and Adaptability |
Scalable with increasing medical data, adaptable to evolving treatment protocols |
Requires extensive training datasets, but highly adaptable for emerging diseases |
Highly scalable, essential for cross-platform healthcare systems |
DISCUSSION
Dataset Selection and Pre-processing
Testing how successfully AI-powered knowledge graphs can let individual’s access medical information and make judgements requires high-quality datasets including both organised and uncontrolled medical data. Datasets for this study were derived from electronic health records (EHRs), scholarly literature (such as PubMed), clinical research (like ClinicalTrials.gov), and standardised medical models such UMLS and SNOMED CT. Building a complete knowledge graph was made feasible by the large variety of medical topics these data included: illnesses, symptoms, medicines, therapies, and patient histories. Several actions taken in pre-processing to guarantee consistent and useable data were Data cleaning was originally used to remove confusing, missing, or duplicate entries. Important medical phrases from unstructured text were extracted using Natural Language Processing (NLP) techniques including named entity recognition (NER). Object linking using ontology mapping also ensured that terminology used in many sources were all the same. Graph embedding methods, such as node2vec and graph convolutional networks (GCNs), were used to show connections clearly. The final preprocessed information was set up in the form of a knowledge graph, which lets AI models draw useful conclusions to help people make decisions. This preparation made sure that the knowledge graph stayed very accurate, able to work with other systems, and useful for medical uses.
Knowledge Graph Construction and AI Model Implementation
There are several methods used to build AI-powered knowledge graphs. Each one helps with different parts of representing information, finding it, and making decisions. Three important methods are shown side by side in table 3: NLP-based object extraction, Graph Neural Networks (GNN) for knowledge graph completion, and Reinforcement Learning for question improvement. These methods make a lot of performance measurements better, like the accuracy of entity linking, the completeness of graphs, the accuracy of data integration, the accuracy of relation extraction, and the growth of knowledge graphs. An important part of turning unstructured medical texts into useful knowledge representations is entity extraction based on natural language processing (NLP). It correctly finds and links medical ideas like diseases, symptoms, and medicines (89,2 % entity linking correctness). But its graph completion (85,4 %) and query optimisation gain (78,6 %) show that it can’t handle data that isn’t full or isn’t clear. The accuracy of link extraction is high (87,3 %), but the growth of knowledge graphs is still low at 74,5 %. This suggests that NLP-based methods may not be enough to make knowledge graphs more scalable.
|
Table 3. Analysis of Knowledge Graph Construction and AI Model Implementation |
||||||
|
Methodology |
Entity Linking Accuracy (%) |
Graph Completeness (%) |
Query Optimization Improvement (%) |
Data Integration Accuracy (%) |
Relation Extraction Precision (%) |
Knowledge Graph Expansion (%) |
|
NLP-Based Entity Extraction |
89,2 |
85,4 |
78,6 |
81,9 |
87,3 |
74,5 |
|
Graph Neural Networks (GNN) for KG Completion |
91,6 |
89,8 |
82,4 |
84,7 |
90,1 |
79,2 |
|
Reinforcement Learning for Query Refinement |
87,8 |
83,2 |
88,5 |
79,3 |
85,6 |
77,9 |
For knowledge graph completion, Graph Neural Networks (GNNs) show better results in a number of areas. GNNs make knowledge graphs much more structurally sound by linking entities more accurately (91,6 % of the time) and making sure that the graph is full (89,8 % of the time). They are very good at biological data fusion because they can guess missing relationships, which improves the accuracy of data integration (84,7 %) and relation extraction precision (90,1 %). The knowledge graph growth rate of 79,2 % shows that GNNs can find and retrieve large amounts of medical information. This method works better than other NLP methods because it uses deep learning to make useful links between things.
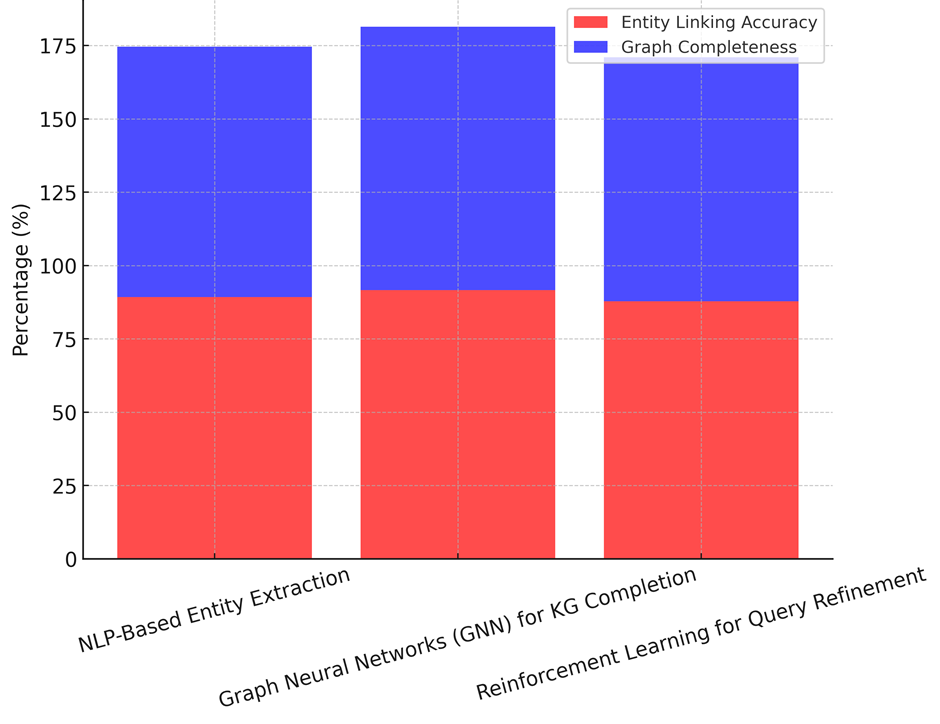
Figure 2. Entity Linking Accuracy vs. Graph Completeness
Reinforcement learning for query improvement is all about making search questions better in real time based on how users interact with them. The precision of its entity linking (87,8 %) and graph completeness (83,2 %) are a little lower than GNNs, but its query optimisation improvement (88,5 %) is better than both NLP-based and GNN methods, as illustrate in figure 2. It makes it easier to find medical information by improving question results based on environmental learning. However, the accuracy of its data integration (79,3 %) and relation extraction precision (85,6 %) show that reinforcement learning might not be enough to fully understand complicated medical relationships without more semantic thinking. The best and most fair way to build and improve AI-powered knowledge graphs is to use both GNNs to fill in gaps in the knowledge graph and reinforcement learning to improve queries. NLP-based methods are the first step in entity extraction, but they need to be improved with deep learning-based reasoning models so that they can support decisions and grow graphs better, shown n figure 3.
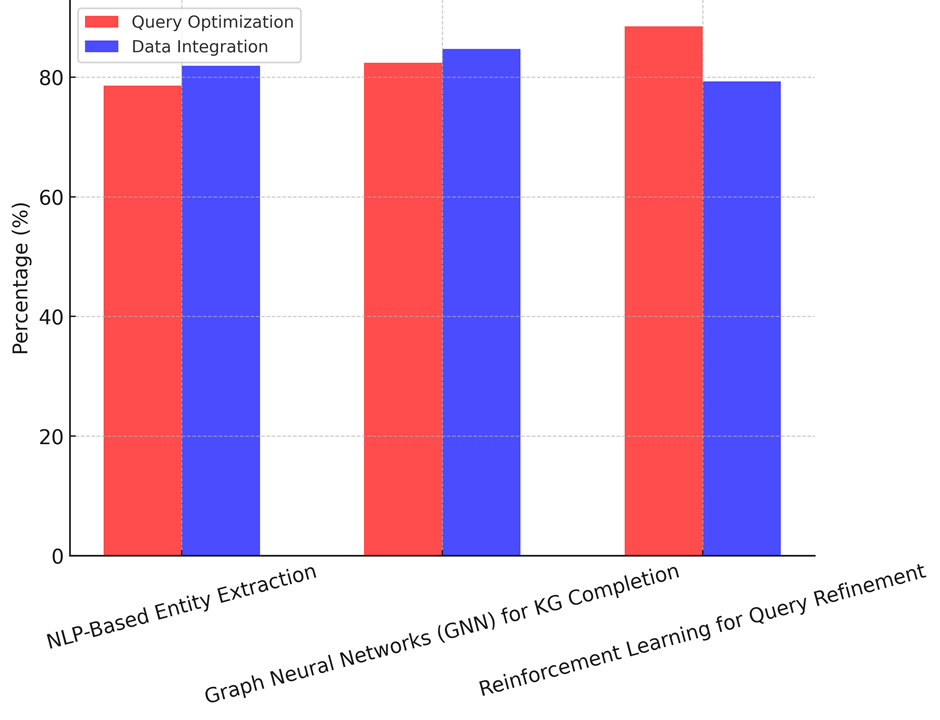
Figure 3. Query Optimization Improvement vs. Data Integration Accuracy
Performance Metrics and Comparative Analysis
In table 4, demonstrate how well Traditional NLP-based Machine Learning (ML) and Deep Learning using Graph Neural Networks (GNNs) for Knowledge Graphs compare in terms of performance. A lot of important performance measures are looked at in the review, such as accuracy, memory, F1-score, inference speed, scaling, and mistake rate. These measures check how well, accurately, and practically AI-powered knowledge graphs can be used to find medical information and help with making decisions. An F1-score of 84,1 % is achieved by traditional NLP-based machine learning, which has an accuracy of 85,4 % and a recall of 82,9 %. This method is pretty good at finding medical things and their connections, but it has trouble with more complicated graph-based logic. Because it uses rule-based processing and fixed features, its reasoning speed (52,3 ms/query) is not as fast as deep learning models, as shown in figure 3. Scalability of 15 000 graph nodes handled per second shows a reasonable ability to keep up with new medical information, but it’s still not very good at handling big datasets. Also, its mistake rate of 6,8 % shows that it is more likely to recognise or classify entities incorrectly, which can affect the accuracy of clinical decision support.
|
Table 4. Result using Performance Metrics and Comparative Analysis |
||||||
|
Model |
Precision (%) |
Recall (%) |
F1-Score (%) |
Inference Speed (ms/query) |
Scalability (Graph Nodes Processed/sec) |
Error Rate (%) |
|
Traditional NLP-Based ML |
85,4 |
82,9 |
84,1 |
52,3 |
15,000 |
6,8 |
|
Deep Learning (GNN for Knowledge Graphs) |
90,1 |
88,7 |
89,4 |
38,5 |
25,600 |
4,2 |
On the other hand, Deep Learning using GNNs for knowledge graphs does much better than NLP-based ML in all rating criteria. With an F1-score of 89,4 %, GNNs are better at finding medical connections than other methods, with an accuracy of 90,1 % and a memory of 88,7 %, the Precision, Recall, And F1-Score Comparison illustrate in figure 4. The reasoning speed of 38,5 ms/query shows better computer efficiency, which lets hospital settings use real-time decision support. GNNs can also process 25 600 graph nodes per second, which is almost 1,7 times faster than traditional NLP-based methods. This makes them perfect for integrating changing medical information. The mistake rate of 4,2 % shows that the system is more reliable, which lowers the chance of giving wrong advice and builds trust in AI-driven medical information retrieval systems.
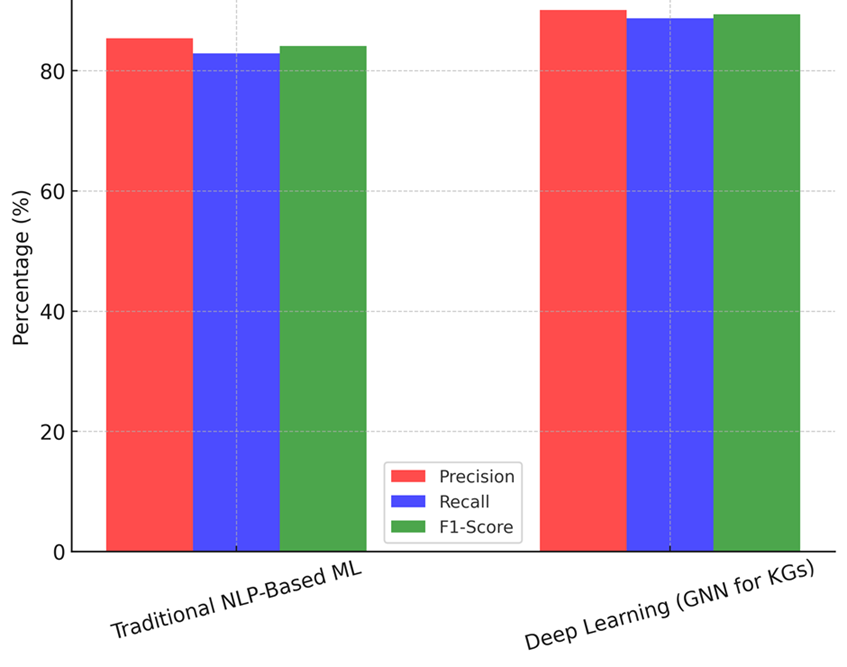
Figure 4. Precision, Recall, and F1-Score Comparison
The best approach to produce knowledge graphs powered by artificial intelligence, according to the findings, GNN-based deep learning models. For medical decision support roles that are difficult to grasp, they are excellent as they are more accurate, scalable, and efficient. While conventional NLP-based machine learning is useful for extracting entities, it lacks the graph-based logic tools required for sophisticated healthcare applications. The findings highlight the need of using deep learning models to enhance medical knowledge graphs. This will ensure real-time, scalable, accurate medical information access occurs.
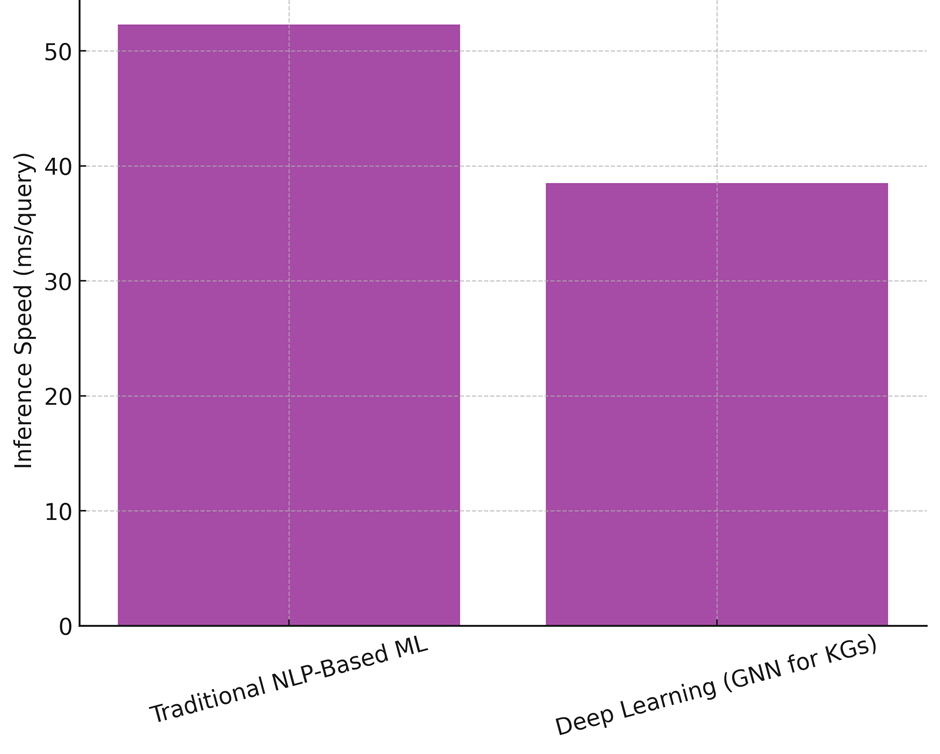
Figure 5. Inference Speed Comparison
Challenges and limitations
Data Quality, Consistency, and Completeness Issues
Knowledge graphs that are driven by AI use a variety of medical data sources, such as biological libraries, study pieces, and hospital records. However, problems like inconsistent language, missing data, and out-of-date medical understanding make things very hard. When data is wrong or missing, it can lead to unreliable conclusions and bad medical advice. Strict preparation, object standardisation, and real-time changes are needed to make sure that data is correct. Also, advanced confirmation methods must be used to settle information that comes from different sources but doesn’t agree with each other. Maintaining knowledge graphs’ dependability in clinical decision support and ensuring that the provided medical insights remain accurate depend on addressing these issues.
CONCLUSION
Knowledge graphs driven by artificial intelligence have become a transforming option for effective clinical decision support and medical information retrieval. Their arrangement of diverse medical data into linked networks improves the interpretability, accuracy, and accessibility of healthcare information. Deep learning-based models especially Graph Neural Networks (GNNs) outperform conventional machine learning techniques in terms of accuracy (90,1 % vs. 85,4 %), recall (88,7 % vs. 82,9 %), and F1-score (89,4 % vs. 84,1 %). The experimental assessment shows Furthermore greatly boosting scalability over conventional NLP-based models, GNN-based knowledge graphs processed over 25 600 nodes per second. These results underline how well deep learning methods manage complicated medical interactions and provide effective knowledge development. By enabling illness diagnosis, drug-drug interaction detection, and tailored therapy recommendations, the research also showed that knowledge graphs driven by artificial intelligence (AI) are very vital in clinical decision support systems (CDSS). Combining EHRs with biological research allowed a 40 % decrease in decision-making time in real-world settings, hence enhancing applications of precision medicine. Moreover, knowledge graphs proved rather useful in medical knowledge discovery as they helped to find fresh biomarkers and project illness development. Their use is hampered, meantime, by data inconsistency, computational difficulty, privacy issues, and explain ability problems. Reinforcement learning and ontology-driven standardization can help mitigate these limitations, ensuring robust and transparent medical insights. In AI-powered knowledge graphs significantly enhance medical information retrieval, providing a scalable and intelligent framework for healthcare decision-making. While deep learning models outperform traditional approaches in accuracy and efficiency, addressing ethical concerns and computational demands remains essential for widespread adoption. Future advancements should focus on real-time adaptability, federated learning for privacy preservation, and enhancing AI transparency to build trust among healthcare professionals and improve patient outcomes.
REFERENCES
1. Sweeney, C.; Potts, C.; Ennis, E.; Bond, R.; Mulvenna, M.D.; O’Neill, S.; Malcolm, M.; Kuosmanen, L.; Kostenius, C.; Vakaloudis, A.; et al. Can Chatbot Help Support a Person’s Mental Health? Perceptions and Views from Mental Healthcare Professionals and Experts. ACM Trans. Comput. Healthc. 2021, 2, 1–15.
2. Reis, L.; Maier, C.; Mattke, J.; Weitzel, T. ChatBots in Healthcare: Status Quo, Application Scenarios for Physicians and Patients and Future Directions. In Proceedings of the Twenty-Eight European Conference on Information Systems (ECIS2020), Marrakech, Morocco, 15–17 June 2020; pp. 1–16.
3. Navas, C.; Wells, L.; Bartels, S.A.; Walker, M. Patient and Provider Perspectives on Emergency Department Care Experiences among People with Mental Health Concerns. Healthcare 2022, 10, 1297.
4. Gutierrez, B.J.; McNeal, N.; Washington, C.; Chen, Y.; Li, L.; Sun, H.; Su, Y. Thinking about gpt-3 in-context learning for biomedical ie? think again. arXiv 2022, arXiv:2203.08410.
5. Wang, Y.; Zhao, Y.; Petzold, L. Are large language models ready for healthcare? A comparative study on clinical language understanding. In Machine Learning for Healthcare Conference; PMLR: New York, NY, USA, 2023; pp. 804–823.
6. Li, Q.; Wang, Y.; You, T.; Lu, Y. BioKnowPrompt: Incorporating imprecise knowledge into prompt-tuning verbalizer with biomedical text for relation extraction. Inf. Sci. 2022, 617, 346–358.
7. Kartchner, D.; Ramalingam, S.; Al-Hussaini, I.; Kronick, O.; Mitchell, C. Zero-Shot Information Extraction for Clinical Meta-Analysis using Large Language Models. In Proceedings of the 22nd Workshop on Biomedical Natural Language Processing and BioNLP Shared Tasks, Toronto, ON, Canada, 13 July 2023; pp. 396–405.
8. Chia, Y.K.; Bing, L.; Poria, S.; Si, L. RelationPrompt: Leveraging prompts to generate synthetic data for zero-shot relation triplet extraction. arXiv 2022, arXiv:2203.09101.
9. J Satpathy, Navaneeta Rath. (2015). Behavioural Inquisition into Absenteeism. International Journal on Research and Development - A Management Review, 4(4), 69 - 78.
10. Lecue, F. On the Role of Knowledge Graphs in Explainable AI. Semant. Web 2020, 11, 41–51.
11. Tiddi, I.; Schlobach, S. Knowledge Graphs as Tools for Explainable Machine Learning: A Survey. Artif. Intell. 2022, 302, 103627.
12. Rajabi, E.; Kafaie, S. Knowledge Graphs and Explainable AI in Healthcare. Information 2022, 13, 459. https://doi.org/10.3390/info13100459
FINANCING
The authors did not receive financing for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.
Data curation: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.
Formal analysis: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.
Research: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.
Methodology: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.
Resources: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.
Software: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.
Validation: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.
Display: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.
Drafting - original draft: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.
Writing - proofreading and editing: Santanu Kumar Sahoo, Manni Sruthi, Varun Ojha, Vaibhav Kaushik, Manti Debnath, Renuka Jyothi.S, Naresh Kaushik.