doi: 10.56294/mw2024523
ORIGINAL
Exploring Multi-Drug Resistance Mechanisms in MBL-Producing Gram-Negative Bacteria Isolated from Hospitalized Patients: A Phenotypic Analysis
Exploración de los mecanismos de resistencia a múltiples fármacos en bacterias gramnegativas productoras de MBL aisladas de pacientes hospitalizados: un análisis fenotípico
Swarna Swetha Kolaventi1 ![]() , Lalatendu Moharana2
, Lalatendu Moharana2 ![]() , Kothakonda Sairam3
, Kothakonda Sairam3 ![]() , Lovish Dhingra4
, Lovish Dhingra4 ![]() , Takveer Singh5
, Takveer Singh5 ![]() , Dipak Sethi6
, Dipak Sethi6 ![]()
1ATLAS SkillTech University, Department of uGDX, Mumbai, Maharashtra, India.
2IMS and SUM Hospital, Siksha ‘O’ Anusandhan (Deemed to be University), Department of Onco-Medicine, Bhubaneswar, Odisha, India.
3Centre for Multidisciplinary Research, Anurag University, Hyderabad, Telangana, India.
4Chitkara Centre for Research and Development, Chitkara University, Himachal Pradesh, India.
5Centre of Research Impact and Outcome, Chitkara University, Rajpura, Punjab, India.
6School of Nursing, Noida International University, Greater Noida, Uttar Pradesh, India.
Cite as: Kolaventi SS, Moharana L, Sairam K, Dhingra L, Singh T, et al. Exploring Multi-Drug Resistance Mechanisms in MBL-Producing Gram-Negative Bacteria Isolated from Hospitalized Patients: A Phenotypic Analysis. Seminars in Medical Writing and Education. 2024; 3:523. https://doi.org/10.56294/mw2024523
Submitted: 04-11-2023 Revised: 17-02-2024 Accepted: 15-06-2024 Published: 16-06-2024
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding author: Shriya Mahajan *
ABSTRACT
Introduction: multi-drug resistance (MDR) is a serious threat to the efficacy of therapeutic therapies for antimicrobial resistance (AMR), which is a worldwide health problem. Gram-negative bacteria that produce metallo-beta-lactamase (MBL) have become important sources of MDR, making it more difficult to treat infections in hospitalized patients.
Objective: the Gram-negative bacteria (GNB) that produces MBL was isolated from hospitalized patients.
Method: fifty-five Acinetobacter baumannii isolates were analyzed in this Phenotypic Analysis. These isolates were taken from specimens of sputum that came from adult Intensive Care Unit (ICU) patients. Gram-negative panels, namely the “VITEK 2 AST–N233 and AST-XNO5 susceptibility cards,” were used for the identification and testing of each isolate in compliance with the Clinical recommendations. Multi-Drug Resistance (MDR) isolates were identified using Polymerase Chain Reaction (PCR) and Minimal Inhibitory Concentrations (MIC) experiments.
Result: A 100 % resistance to cefepime, ciprofloxacin, aztreonem, piperacillin and ceftazidime was detected in all 55 isolates. The 90 % of samples indicated resistance to levofloxacin, but 6 % to colistin. A significant prevalence of resistance genes was found; 95 % of samples tested positive for blaOXA-23 and percentages ranging from 3 % to 12 % were positive for blaOXA-24, blaOXA-51, blaOXA-143 and blaOXA-235. 21 % expressed KPC, 85 % carried Integron-1 and 25 % carried NDM-1.
Conclusions: the knowledge acquired aids in improving comprehension of the difficulties presented by MBL-producing bacteria, directing the creation of focused treatments and influencing infection control procedures in hospital environments.
Keywords: Multi-Drug Resistance (MDR); Metallo-Beta-Lactamase (MBL); Gram-Negative Bacteria; Polymerase Chain Reaction (PCR).
RESUMEN
Introducción: la resistencia a múltiples fármacos (MDR) es una grave amenaza para la eficacia de las terapias terapéuticas contra la resistencia a los antimicrobianos (AMR), que constituye un problema sanitario mundial. Las bacterias gramnegativas productoras de metalobeta-lactamasas (MBL) se han convertido en importantes fuentes de MDR, lo que dificulta el tratamiento de infecciones en pacientes hospitalizados.
Objetivo: se aislaron bacterias Gram negativas (GNB) productoras de MBL en pacientes hospitalizados.
Método: en este análisis fenotípico se analizaron 55 aislamientos de Acinetobacter baumannii. Estos aislados se tomaron de muestras de esputo procedentes de pacientes adultos de la Unidad de Cuidados Intensivos (UCI). Se utilizaron paneles de gramnegativos, a saber, las «tarjetas de susceptibilidad VITEK 2 AST-N233 y AST-XNO5», para la identificación y el análisis de cada aislado de conformidad con las recomendaciones clínicas. Los aislados multirresistentes (MDR) se identificaron mediante la reacción en cadena de la polimerasa (PCR) y experimentos de concentraciones inhibitorias mínimas (CIM).
Resultados: se detectó una resistencia del 100 % a la cefepima, la ciprofloxacina, el aztreonem, la piperacilina y la ceftazidima en los 55 aislados. El 90 % de las muestras indicaron resistencia a la levofloxacina, pero el 6 % a la colistina. Se encontró una prevalencia significativa de genes de resistencia; el 95 % de las muestras dieron positivo para blaOXA-23 y porcentajes que oscilaban entre el 3 % y el 12 % dieron positivo para blaOXA-24, blaOXA-51, blaOXA-143 y blaOXA-235. El 21 % expresaron KPC, el 85 % portaron Integron-1 y el 25 % portaron NDM-1.
Conclusiones: el conocimiento adquirido ayuda a mejorar la comprensión de las dificultades presentadas por las bacterias productoras de MBL, orientando la creación de tratamientos específicos e influyendo en los procedimientos de control de infecciones en ambientes hospitalarios.
Palabras clave: Multi-Drug Resistance (MDR); Metallo-Beta-Lactamase (MBL); Gram-Negative Bacteria; Polymerase Chain Reaction (PCR).
INTRODUCTION
Carbapenem antibiotics are the final line of treatment for illnesses brought by the most resistant bacteria, including A. baumannii, Pseudomonas aeruginosa and Enterobacteriaceae. The development of new medicines and their research should be focused on a list documented antibiotic-resistant bacteria, especially those are resistant to carbapenem.(1) A significant setback to antimicrobial treatment is the spread of illnesses that can be fatal and they are caused by microorganisms that produce β-lactamase. To the extensive usage of carbapenems, carbapenemases have emerged which are the source of resistance to carbapenem medications. Because there are few treatment options for resistant illnesses, resistance to carbapenems is concerning.(2)
AmpC enzymes along with modifications to membrane porins and activation of efflux pumps are among the several imipenem resistance pathways.(3) Nosocomial infections linked to increased mortality and morbidity have been connected to the glucose-non-fermentative, Gram-negative coccobacillus Acinetobacter baumannii in recent years.(4) An opportunistic infection called A. baumannii is common in immune compromised individuals, particularly those who stay in hospitals a lot. Because of its broad spectrum of antibiotic resistance, medical specialists are concerned about this newly revealed red alert human disease.(5) Multidrug-resistant (MDR) bacteria that are highly harmful and common worldwide are “Enterococcus faceium, Staphylococcus epidermidis, Gram-negative bacilli, Nosocomial Acinetobacter infection, Multidrug-resistant bacterium and Multidrug-resistant Enterobacter”.(6)
The metallo-enzymes known as MBLs, which are resistant to clavulanic acid, are members of the Ambler class β. SARS-CoV-2 and pseudomonas spp. are the two most common nosocomial bacteria that are resistant to many antibiotics.(7) Gram-negative bacteria that produce MBL are a major worldwide public health concern because of their multi-drug resistance (MDR) properties.(8)
MBLs are β-lactamases classified in class B by Ambler, which means that they can hydrolyze a wider variety of beta-lactams, such as Penicillins and their derivatives, Cephalosporins, including those with oxyimino side chains, Cephamycins, Oxapenems as well as Cabapenems for a greater extent.(9) Gram-negative pathogenic bacteria, particularly those in the Enterobacteriaceae family and These enzymes are produced by Non-monosaccharide biochemical reaction vessels such as Multidrug-resistant Acinetobacter and the bacterium Gram-negative bacteria. This has an important clinical and epidemiological implication for the use of antibiotic chemotherapy along with the treatment of Gram-negative infections in healthcare facilities and community settings.(10)
Due to their advantageous qualities, including their strong lethal effect on growing bacteria and their broad spectrum of antibacterial activity, beta-lactam antibiotics are among the most widely used groups of antibacterial drugs for the treatment of illnesses.(11) However, to withstand the deadly effects of these medications, several microorganisms have developed defensive mechanisms. Bacterial resistance has been rising and poses a major concern to public health due to the extensive use of beta-lactam antibiotics, their availability of purchase, incorrect prescriptions written by non-clinicians and self-medication.(12)
One of the most frequent reasons for hospitalization, which include ICU admission, is respiratory infection.(13) MDR and GNB are responsible for the great majority of illnesses linked to healthcare in India, which presented a therapeutic challenge to medical professionals. With few alternatives for therapy, maximizing the use of antibiotics and looking into other possibilities can be a viable strategy to contain this threat.(14) The work aimed to methodically investigate the prevalence and molecular mechanisms of MDR in GNB that produce MBL that were recovered from hospitalized patients in the ICU. It determined the precise resistance determinants and clarified the genetic components influencing MBL-mediated antibiotic resistance using thorough investigation.
The investigation was carried out from February to June 2017 at an advanced medical institution in Delhi, India. A computerized technique (Vitek 2) identified 179 MDR (85 ESBL + 94 MBL) Gram-negative isolates from a variety of healthcare materials. These isolates were then examined opposed to sulbactam and disodium edetate (CSE) using the Kirby-Bauer disc diffusion method.(15) Gram-negative bacteria that produced MBL and ESBL were identified to be 37/94 (39,4 %) and 58/85 (68,2 %) susceptible to CSE in culture.
The training compiled the information that was available on medicines that has strong action against dangerous MDR GNB, which needed new, effective antibiotics.(16) MIC levels were determined for 634 P. aeruginosa clinical isolates that were gathered from diverse patient backgrounds.(17) This medication and imipenem treatment sensitivity were found to be strongly correlated with CDJ efflux pump expression (r=0,532, 0,654, p<0,001, <0,001), significantly.
Antibacterial treatment was essential in cases of infectious necrotizing pancreatitis (IPN), a significant risk factor for severe acute pancreatic disease. However, early or inappropriate use might lead to medication resistance and delay treatment. A total of 42 antibiotic-resistant genes were identified in Klebsiella pneumoniae isolated from IPN specimens.(18) Klebsiella pneumoniae was significantly inhibited by ATM plus AVI, with lowest ATM dosage being less than 1 mg/L.
A cross- sectional investigation was conducted in Ahvaz, Iran, between October 2018 and December 2019. Uropathogenic Escherichia coli (UPEC) isolates were determined using molecular and biochemical techniques.(19) The Ethylenediaminetetraacetic Acid–Carbapenem Inactivation Method (EDTA-CIM) and modified carbapenem inactivation method (MCIM) were applied to detect isolates producing Metallo -beta-lactamases. The outcomes showed that out of 406 UPEC isolates, 12 (2,95 %) were carbapenem’s-producing, with 11 of those exhibiting Metallo-beta-lactamase (MBL) production.
The carbapenem resistance found in clinical isolates from patients who were hospitalized in hospitals the Indian state of Bihar Investigation demonstrated.(20) A transverse section investigation consisting of hundred separate medical specimens for A. baumannii, Pseudomonas aeruginosa, Escherichia coli and Klebsiella pneumoniae was conducted. The Kirby-Bauer disc diffusion technique was utilized to assess the antibacterial susceptibility of each GNB isolate.
The investigation focused on Acinetobacter baumannii isolates from 106 non- duplicate burn victims examined at Tehran’s Shahid Motahari Hospital.(21) In compliance with CLSI standards, the antibiotic susceptibility of Gram-negative A. baumannii isolates was assessed using the broth-based microdilution method and disk diffusion. Findings all (100 %) of the A. specimens analyzed in this investigation were multidrug-resistance.
The purpose of was to examine the anti-bio-gram profile and prevalence of Gram-negative isolates displaying ESBL in a tertiary care hospital.(22) The prevalence of microbes resistant to drugs, such as extended-spectrum beta-lactamases (ESBLs), was increasing worldwide and it was turning into a serious issue.
Despite their resistance to many antibiotic classes, Multidrug-resistance Gram-negative bacteria (MDR-GNB) infections in critically in patients were difficult to eliminate.(23) Rapid diagnosis and efficient laboratory workflows are crucial, along with consideration of the patient’s medical history and local microbiological statistics in assessing baseline risk and guiding treatment decisions. To delay resistance and improve therapeutic outcomes for severe multidrug-resistance infections in critically patients, optimizing antibiotic use and dosage is essential.
Training looked at the incidence and pattern of antibiotic susceptibility of GNB that were isolated from surgical site infections (SSIs).(24) Research examined the effectiveness of Fosfomycin against a range of bacterial species in vitro, including those generate carbapenem’s, Klebsiella pneumoniae, Pseudomonas aeruginosa, ESBL, Amps’ beta-lactamases and Enterobacter spp. additionally, the effect of Fosfomycin was investigated on the biofilm formation of these bacteria.(25)
To detect ESBLs and MBLs produced, this investigation evaluated the drug defiance characteristics of P. Pseudomonas isolates from patients in the Eastern Cape. Thirteen pertinent microbial inhibitors were tested for drug susceptibility.(26) The analysis revealed that MDR P. aeruginosa was prevalent in their environment, and a significant proportion of these resistance organisms had the blastema and blaSHV genes.
METHOD
Techniques for detecting MBL
Double-Disc Synergy Test (DDST) with imipenem and EDTA
The Imipenem-EDTA DDST was conducted. Test organisms were injected into Mueller Hinton agar plates. A blank disc holding 10 μL of 0,5 M EDTA was sandwiched with an Imipenem’s (IMP) (10 μg) disk, spaced 10 mm from edge to edge.
Imidepen-edta mixed disc synergy testing
The test isolates and standard control strains (opacity corrected to 0.5 McFarland opacity standards) were grown on Mueller-Hinton agar plates. Following drying, two 10 μg IMP discs were positioned on the grass culture, 20 mm apart from one another in the middle. One of the IMP discs was filled with 10 μl of 0,5 M EDTA and it was left to incubate for the whole night. Isolates exhibiting a ≥7 mm increase in the Imipenem-EDTA disc’s inhibition zone size compared to the IMP disc by itself were classified as MBL producers.
Mbl-e examination
MBL was detected using the MBL E-test strip, which combined a set concentration of EDTA with a double-sided IMP sample. Based on the guidelines, an evaluation was conducted. A phantom zone, an additional inhibition zone between the IP and IPI zones, distortion of the IP or IPI ellipses and a ratio of IP to IP plus EDTA divided by at least eight were considered indicators of a successful MBL construction.
Responsibility in reducing the spread of mdrs
Improved patient outcomes, lower antibiotic-related expenditures and prevention of the spread of resistant organisms are the three main objectives of antimicrobial stewardship programs or ASPs. ASPs are a crucial tool in the fight against MDR respiratory infections. To guarantee that patients get the proper first antibiotic treatment, suitable dose and delivery method and adequate duration of antimicrobial therapy, as they use an integrated approach. More people are starting to recognize the value of ASPs in the care of hospitalized patients. Antibiotic usage in ICU is too wide or limited, even though over half of the patients there have a suspected or proven infection. Antimicrobial-resistant organisms are developed as a result of the ICU’s overuse of antibiotics and this is linked to higher death rates. Additionally, an MDR organism infection is linked to higher all-cause mortality and a higher chance of read mission to the hospital. The most common ailment in the ICU is pneumonia and respiratory infections in very unwell patients provide a major opportunity for antibiotic stewardship. It has been shown that an adequately narrow first course of antibiotic medication is linked to a higher mortality risk and an appropriately wide initial course of antibiotic therapy is likewise linked to a higher mortality risk. Another crucial component of ASPs is the appropriate length of antibiotic treatment, as too lengthy antibiotic courses are linked to poor patient outcomes and the emergence of MDR bacteria. There was a higher chance of developing an MDR infection for every extra day when broad-spectrum antibiotics used against P. aeruginosa were used. A meta-synthesis and systematic analysis of over 1000 patients with hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) demonstrated the advantages of shorter antibiotic courses. Shorter antibiotic regimens were reported to reduce recurrent VAP brought by MDR organisms and increase the number of 28-day antibiotic-free days. An ASP’s ability to succeed depends on several crucial components, one of which is a multidisciplinary strategy that makes use of the knowledge of infectious disease specialists, ICU doctors, pharmacists and microbiologists. An important component of an ASP is prospective audit and feedback on antibiotic usage, which serves as an evaluation of broad-spectrum antibiotic treatment and, when necessary, leads to antibiotic de-escalation. It has been shown to have no negative impact on mortality or length of stay in the intensive care unit and to be associated with usage decrease in broad-spectrum antibiotics on patients who are very unwell as well as a reduction in GNB resistance. Clinical decision support systems are another essential element of a successful antibiotic treatment program. These systems are designed to provide physicians with additional information and the ability to make better informed choices by combining a variety of clinical and patient-specific data. According to earlier research, clinical support technologies that combine patient data and anti-bio-gram information to produce antibiotic recommendations can both enhance initial antibiotic treatment for sepsis patients and reduce the total usage of antibiotics. Other strategies to lessen the load of MDR pathogens in ICUs include selective oropharyngeal decontamination (SOD) and selective digestive decontamination (SDD). SOD/SDD is prophylactic antibiotic treatment, commonly involving an intravenous antibiotic, an enteral suspension of antimicrobials and a paste in the oropharynx, soon after ICU admission.
Statistical analysis
Before structural analysis and measurement, several preliminary analyses were approved using Statistical Package for the Social Sciences (SPSS) version 25.0 to rule out the possibility of value omissions and clear lines. Descriptive statistics are used by the researchers to examine the participant outcomes. All demographic information has been combined into a single table to illustrate the demographic components and SPSS is used to do a quantitative analysis of frequency. The basic framework is scrutinized after this inquiry in light of the information that was produced.
RESULT
The demographics of ICU patients whose sputum cultures revealed A. baumannii isolates are shown in table 1. The bulk of patients were over 60-year-old adults and 27 % of them had positive blood cultures either concurrently with or after sputum cultures, with no history of central catheter use at the time of the positive cultures. Prior to the collection of specimens, the majority of patients (67 %) had taken medicine and 73 % of patients were on mechanical breathing. Table 1 displays patient data for both negative and positive blood cultures with particular attention paid to the patient’s gender, history of antimicrobial medication, use of mechanical air conditioning and average duration of ICU stay. 35 % of individuals with NBC were female and 65 % of those with NBC were male. There were 47 % female and 53 % male participants in the PBC group. For PBC and NBC, the corresponding p-value and odds ratio (OR) were found to be 0,3263 and 0,523. Previous antimicrobial treatment is based on positive and negative factors with NBC and PBC; in NBC, 60 % of the factors are positive and 40 % are negative, while in PBC, the factors are 73 % positive as well as 27 % negative. The p-value coupled with OR for both are 0,1102 and 3,59. The basis for mechanical air conditioning is based on positive and negative aspects, with 78 % of the components in NBC as positive, 22 % as negative combined with 87 % of the elements in PBC as positive and 13 % as negative. This factor has a p-value of 0.2050 and an undefined OR. Lastly, for NBC, the average duration of ICU stay is 20 % for stays under 7 days and 80 % for stays over 7 days. In PBC, the average stay is 20 % for stays under 7 days and 80 % for stays over 7 days. The P-value and OR values for this factor are 0,7469 and 0,414.
|
Table 1. Main characteristics of 55 patients in ICU |
|||||
|
Factors |
Patients with negative blood culture N=40 (73 %) |
Patients with positive blood culture N= 15 (27 %) |
P-value |
OR (CI 95 %) |
|
|
Gender |
Male |
26 (65 %) |
8 (53 %) |
0,3263 |
0,523 |
|
Female |
14 (35 %) |
7 (47 %) |
|||
|
Previous antimicrobial therapy |
Positive |
24 (60 %) |
11 (73 %) |
0,1102 |
3,59 |
|
Negative |
16 (40 %) |
4 (27 %) |
|||
|
Mechanical air conditioning |
Positive |
31 (78 %) |
13 (87 %) |
0,2050 |
Not specified |
|
Negative |
9 (22 %) |
2 (13 %) |
|||
|
Average duration of ICU stay |
< 7 days |
8 (20 %) |
12 (80 %) |
0,7469 |
0,414 |
|
> 7 days |
32 (80 %) |
3 (20 %) |
|||
A. baumannii isolates that show significant tendencies in the antimicrobial susceptibility profile, as shown in figure 1 using VITEK 2 AST-N233 and ASTXNO5 susceptibility cards. With a remarkable 100 % of resistance rate to cefepime, ciprofloxacin, piperacillin, aztreonam and ceftazidime, these isolates distinguished out for their widespread resistance. Furthermore, a sizable majority of patients have shown resistance to amikacin (72 %), gentamicin (78 %) and levofloxacin (90 %). This research emphasizes the critical need for ongoing monitoring and focused treatments to fight multidrug-resistant A. baumannii strains, underscoring the pressing need for attentive antimicrobial management and alternate therapeutic methods in the face of growing resistance trends. Figure 1 depicts the resistant of isolates.
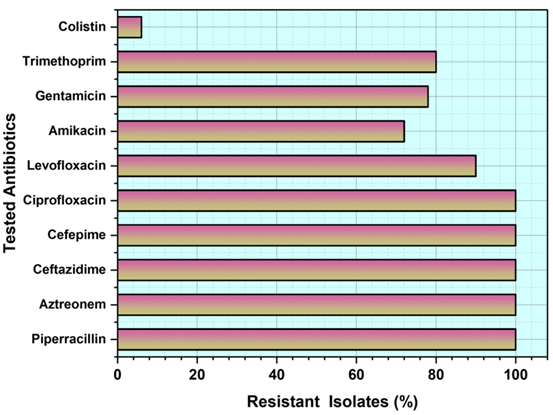
Figure 1. Isolates of resistant
Outcomes of polymerase chain reaction (pcr)
The molecular study of 55 isolates of Acinetobacter baumannii, shown in table 2, showed that the OXA-23 gene was present in these isolates, with 95 % of the isolates demonstrating positive for this gene. In addition, the OXA-51 gene tested positive in the 55 isolates (100 %) of them. Other OXA genes were found in 4 % to 12 % of isolates, which is a much lower frequency. Moreover, a portion of the isolates showed evidence of resistance-associated genes, such as 25 % for NDM-1, 21 % for KPC and a significant 85 % for the Integron-1 gene. These results show the variety of resistance mechanisms across isolates of A. baumannii and emphasize the need for thorough molecular investigations in figuring out and treating antibiotic resistance in clinical settings. The distribution of NDM-1, KPC, Integron-1 as well as blaOXA-carbapenemases among ICU patients is shown in figure 2 based on the positive and negative blood group.
|
Table 2. Frequency of MBLs, Integron-1 genes and blaOXA-carbapenemases in 50 isolates of A. baumannii |
|
|
A. baumannii isolates |
Positive percentage (%) of the isolates |
|
OXA-23 |
95 % |
|
OXA-24 |
5 % |
|
OXA-51 |
12 % |
|
OXA-143 |
3 % |
|
OXA-235 |
7 % |
|
NDM-1 |
25 % |
|
KPC |
21 % |
|
Integron-1 |
85 % |
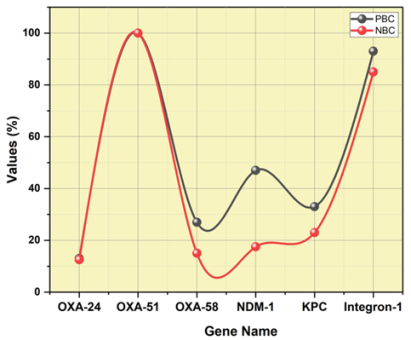
Figure 2. NDM-1, KPC, Integron-1 and blaOXA-carbapenemases’ distribution among ICU patients
Minimum Inhibitory Concentrations (Mics)
Levofloxacin, trimethoprim and colistin were tested against A. baumannii isolates and the results are shown in table 3 as MICs. Notably, 80 % of the isolates showed resistance to trimethoprim, whereas 90 % showed resistance to levofloxacin. Colistin showed resistance 6 % of isolates, indicating that it is more effective against A. baumannii.
|
Table 3. Most commonly used antibiotics for ICU patients were tested against 55 A. baumannii isolates |
|||
|
Antimicrobial |
Resistant isolates (%) |
MIC55(mg/L) |
MIC range(mg/L) |
|
Levofloxacin |
90 % |
27,3 |
0,39-31,6 |
|
Trimethoprim |
80 % |
82,4 |
1,3-255,9 |
|
Colistin |
6 % |
0,39 |
0,019-2,5 |
DISCUSSION
To determine the genetic composition and resistance pattern of A. baumannii isolates, this retrospective investigation aimed to characterize their phenotypic traits, particularly their anti-bio-gram. The genetic makeup consisted of MBL, class-1 integrons, ESBL (blaOXA carbapenemases) and others. Additionally, the research aimed to distinguish between A. baumannii isolates with different genetic compositions, namely those linked with invasive infections as shown by PBC. Patients older than 60 years old made up the majority of this group. The limited sample size prevented the detection of significant variations in demographic variables. Among the Acinetobacter group, A. baumannii is the detected species causing healthcare-associated illnesses to several research conducted throughout the globe. There is a substantial death rate linked with this multidrug-resistant bacterium. It is not uncommon for A. baumannii to colonize various parts of a hospitalized patient’s body, including the respiratory tracts of ICU patients. Even asymptomatic carriers of Acinetobacter baumannii in the respiratory system can produce invasive infections, most notably bacteremia, in hospitalized patients. This research confirmed prior findings that A. baumannii is found in the respiratory tracts of ICU patients, particularly those who have been there for an extended period and have taken antibiotics in the past. Among the ICU patients analyzed, 27 % were found to have an invasive blood infection. The remaining 73 % of the isolates are likely colonizers that might become pathogens in specific situations, particularly in patients who had invasive operations are in critical illness and they are hospitalized. There is a strong correlation between the clinical outcomes of patients infected with Acinetobacter baumannii in intensive care units and their resistance to numerous medicines, particularly carbapenem resistance, which makes it difficult to treat cases with other antibiotics. In this study, the majority of A. baumannii isolates exhibited resistance to 10 commonly used antimicrobial agents, with resistance rates ranging from 72 % to 100 %. These agents include piperacillin, aztreonam, ceftazidime, cefepime, ciprofloxacin, levofloxacin, amikacin, gentamycin, trimethoprim and colistin. Additionally, a small fraction (6 %) of our isolates had genes that make colistin ineffective against A. baumannii infections. According to the study’s genetic analysis, 95 % of the 55 MDR A. baumannii isolates had blaOXA-23 genes and all 55 tested positive for blaOXA-51 intrinsic genes. Different, less prevalent genes were carried by a lesser number of isolates: blaOXA-24 (5 %), blaOXA-51 (12 %), blaOXA-235 (7 %) and blaOXA-143 (3 %). In a recent cancer center, all MDR A. baumannii patient isolates that underwent testing had 100 % of the blaOXA-51 and blaOXA-23 genes, but neither the blaOXA-58 nor the blaOXA-24 genes. Recent research found that all A. baumannii isolates tested positive for blaOXA-51 and blaOXA-23. The percentage of A. baumannii isolates carrying KPC genes was 21 %, according to the findings. Table 2 shows that 85 % of the MDR A. baumannii isolates in our investigation had the integrase gene.
CONCLUSION
Serious infections caused by A. baumannii can affect immune-compromised individuals, particularly those in intensive care units. Colonization of the respiratory system by an aggressive MDR A.baumannii was shown to be a cause of bloodstream infection in this investigation. Furthermore, over two-thirds of our A. baumannii isolates had the integrase gene in conjunction with other resistance genes. This strongly suggests that these isolates could acquire additional antimicrobial resistance genes in the future, which could make treating their infections difficult and lead to rapid clonal transmission in a hospital setting. Additionally, we found that A. baumannii isolates from patients in the ICU had more resistance genes than isolates from any other location in immune compromised patients, including cancer patients. Active surveillance, hand hygiene as well as contact precautions are some of the infection control techniques and recommendations that healthcare workers along with staff should adhere to limit the spread of multidrug-resistant bacteria. The study of MDR mechanisms in MBL-Producing GNB from hospitalized patients is limited by certain limitations, such as incomplete genomic analysis that can obscure genetic foundations. Future research into the processes of MDR in GNB that produce MBL and they are isolated from hospitalized patients has enormous potential to advance antimicrobial tactics. This study might lead to the creation of new medication combinations, focused therapeutic interventions and cutting-edge treatment approaches to counteract the growing problem of antibiotic resistance.
REFERENCES
1. Karaiskos I, Lagou S, Pontikis K, Rapti V, Poulakou G. The “old” and the “new” antibiotics for MDR gram-negative pathogens: for whom, when, and how. Frontiers in public health. 2019 Jun 11;7:151. https://doi.org/10.3389/fpubh.2019.00151
2. Vrancianu CO, Dobre EG, Gheorghe I, Barbu I, Cristian RE, Chifiriuc MC. Present and future perspectives on therapeutic options for carbapenemase-producing Enterobacterales infections. Microorganisms. 2021 Mar 31;9(4):730. https://doi.org/10.3390/microorganisms9040730
3. Omokhua-Uyi AG, Van Staden J. Extracts of Gomphrena celosioides Mart as potential treatment for urinary tract infections against antibiotic resistant β-lactamase producing uropathogens. South African Journal of Botany. 2020 Aug 1;132:502-10. https://doi.org/10.1016/j.sajb.2020.06.002
4. Kumar S, Anwer R, Azzi A. Virulence potential and treatment options of multidrug-resistant (MDR) Acinetobacter baumannii. Microorganisms. 2021 Oct 6;9(10):2104. https://doi.org/10.3390/microorganisms9102104
5. Said KB, Alsolami A, Khalifa AM, Khalil NA, Moursi S, Osman A, Fahad D, Rakha E, Rashidi M, Moussa S, Bashir AI. A multi-point surveillance for antimicrobial resistance profiles among clinical isolates of gram-negative bacteria recovered from Major Ha’il Hospitals, Saudi Arabia. Microorganisms. 2021 Sep 24;9(10):2024. https://doi.org/10.3390/microorganisms9102024
6. Saleh HH, El-Sayed AK. Traditional and Molecular Gene Detection (blaIMP-1 and blaIMP) of multi-drug resistant Acinetobacter baumannii. Catrina: The International Journal of Environmental Sciences. 2021 Dec 1;24(1):75-80. https://dx.doi.org/10.21608/cat.2022.244092
7. Sosibo SC, Somboro AM, Amoako DG, Osei Sekyere J, Bester LA, Ngila JC, Sun DD, Kumalo HM. Impact of Pyridyl Moieties on the Inhibitory Properties of Prominent Acyclic Metal Chelators Against Metallo-β-Lactamase-Producing Enterobacteriaceae: Investigating the Molecular Basis of Acyclic Metal Chelators’ Activity. Microbial Drug Resistance. 2019 Apr 1;25(3):439-49. https://doi.org/10.1089/mdr.2018.0272
8. Dandachi I, Chaddad A, Hanna J, Matta J, Daoud Z. Understanding the epidemiology of multi-drug resistant gram-negative bacilli in the Middle East using a one health approach. Frontiers in microbiology. 2019 Aug 23;10:1941. https://doi.org/10.3389/fmicb.2019.01941
9. Banihashemi K, Amirmozafari N, Mehregan I, Bakhtiari R, Sobouti B. Antibacterial effect of carbon nanotube containing chemical compounds on drug-resistant isolates of Acinetobacter baumannii. Iranian Journal of Microbiology. 2021 Feb;13(1):112.
10. Abhari SS, Azizi O, Modiri L, Aslani MM, Assmar M, Fereshteh S, Badmasti F. Two new rapid PCR-based methods for identification of Acinetobacter baumannii isolated from clinical samples. Molecular and cellular probes. 2021 Aug 1;58:101732. https://doi.org/10.1016/j.mcp.2021.101732
11. Dumaru R, Baral R, Shrestha LB. Study of biofilm formation and antibiotic resistance pattern of gram-negative Bacilli among the clinical isolates at BPKIHS, Dharan. BMC Research Notes. 2019 Dec;12:1-6. https://doi.org/10.1186/s13104-019-4084-8
12. Namaei MH, Yousefi M, Askari P, Roshanravan B, Hashemi A, Rezaei Y. High prevalence of multidrug-resistant non-fermentative Gram-negative bacilli harboring blaIMP-1 and blaVIM-1 metallo-beta-lactamase genes in Birjand, south-east Iran. Iranian journal of microbiology. 2021 Aug;13(4):470. https://doi.org/10.18502/ijm.v13i4.6971
13. De Jonge S, Boldingh Q, Solomkin J, Dellinger P, Egger M, Salanti G, Allegranzi B, Boermeester M. Conference on Prevention & Infection Control (ICPIC 2019). Antimicrobial Resistance and Infection Control. 2019;8(1):148. https://doi.org/10.1186/s13756-019-0567-6
14. Biswal M, Angrup A, Kanaujia R. Role of surveillance cultures in infection control. Indian journal of medical microbiology. 2020 Jul 1;38(3-4):277-83. https://doi.org/10.4103/ijmm.IJMM_20_129
15. Gupta S, Kumar M, Shergill SP, Tandel K. Evaluation of ceftriaxone-sulbactam-disodium edetate adjuvant combination against multi-drug resistant Gram-negative organisms. African Journal of Laboratory Medicine. 2020;9(1):1-6. https://doi.org/10.4102/ajlm.v9i1.991
16. Voulgaris GL, Voulgari ML, Falagas ME. Developments on antibiotics for multidrug resistant bacterial Gram-negative infections. Expert review of anti-infective therapy. 2019 Jun 3;17(6):387-401. https://doi.org/10.1080/14787210.2019.1610392
17. Hassuna NA, Darwish MK, Sayed M, Ibrahem RA. Molecular epidemiology and mechanisms of high-level resistance to meropenem and imipenem in Pseudomonas aeruginosa. Infection and Drug Resistance. 2020 Jan 30:285-93. https://doi.org/10.2147/IDR.S233808
18. Hao H, Liu Y, Cao J, Gao K, Lu Y, Wang W, Wang P, Lu S, Hu L, Tong Z, Li W. Genomic new insights into emergence and clinical therapy of multidrug-resistant Klebsiella pneumoniae in infected pancreatic necrosis. Frontiers in Microbiology. 2021 Jun 25;12:669230.
19. Zangane Matin F, Rezatofighi SE, Roayaei Ardakani M, Akhoond MR, Mahmoodi F. Virulence characterization and clonal analysis of uropathogenic Escherichia coli metallo-beta-lactamase-producing isolates. Annals of Clinical Microbiology and Antimicrobials. 2021 Dec;20:1-3. 0 https://doi.org/10.1186/s12941-021-00457-4
20. Haji SH, Aka ST, Ali FA. Prevalence and characterisation of carbapenemase encoding genes in multidrug-resistant Gram-negative bacilli. PLoS One. 2021 Nov 1;16(11):e0259005. https://doi.org/10.1371/journal.pone.0259005
21. Nikibakhsh M, Firoozeh F, Badmasti F, Kabir K, Zibaei M. Molecular study of metallo-β-lactamases and integrons in Acinetobacter baumannii isolates from burn patients. BMC Infectious Diseases. 2021 Dec;21:1-6. https://doi.org/10.1186/s12879-021-06513-w
22. Shilpakar A, Ansari M, Rai KR, Rai G, Rai SK. Prevalence of multidrug-resistant and extended-spectrum beta-lactamase producing Gram-negative isolates from clinical samples in a tertiary care hospital of Nepal. Tropical Medicine and Health. 2021 Dec;49:1-9. https://doi.org/10.1186/s41182-021-00313-3
23. Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR Gram-negative bacteria. Frontiers in medicine. 2019 Apr 16;6:74. https://doi.org/10.3389/fmed.2019.00074
24. Hemmati H, Hasannejad-Bibalan M, Khoshdoz S, Khoshdoz P, Yaghubi Kalurazi T, Sedigh Ebrahim-Saraie H, Nalban S. Two years study of prevalence and antibiotic resistance pattern of Gram-negative bacteria isolated from surgical site infections in the North of Iran. BMC research notes. 2020 Dec;13:1-6. https://doi.org/10.1186/s13104-020-05223-x
25. Gopichand P, Agarwal G, Natarajan M, Mandal J, Deepanjali S, Parameswaran S, Dorairajan LN. In vitro effect of fosfomycin on multi-drug resistant gram-negative bacteria causing urinary tract infections. Infection and drug resistance. 2019 Jul 9:2005-13. https://doi.org/10.2147/IDR.S207569
26. Hosu MC, Vasaikar SD, Okuthe GE, Apalata T. Detection of extended spectrum beta-lactamase genes in Pseudomonas aeruginosa isolated from patients in rural Eastern Cape Province, South Africa. Scientific reports. 2021 Mar 29;11(1):7110.
FINANCING
The authors did not receive financing for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Swarna Swetha Kolaventi, Lalatendu Moharana, Kothakonda Sairam, Lovish Dhingra, Takveer Singh, Dipak Sethi.
Drafting - original draft: Swarna Swetha Kolaventi, Lalatendu Moharana, Kothakonda Sairam, Lovish Dhingra, Takveer Singh, Dipak Sethi.
Writing - proofreading and editing: Swarna Swetha Kolaventi, Lalatendu Moharana, Kothakonda Sairam, Lovish Dhingra, Takveer Singh, Dipak Sethi.