doi: 10.56294/mw2024528
ORIGINAL
Assessment of the Risk Factors for Gastrointestinal Bleeding Among Individuals with Left Ventricular Support Machines
Evaluación de los factores de riesgo de hemorragia gastrointestinal en personas con máquinas de asistencia ventricular izquierda
Dheeravath Raju1 ![]() , Abhinav Mishra2
, Abhinav Mishra2 ![]() ,
Rahul Thakur3
,
Rahul Thakur3 ![]() ,
Avir Sarkar4
,
Avir Sarkar4 ![]() ,
Swarna Swetha Kolaventi5
,
Swarna Swetha Kolaventi5 ![]() ,
Jimmy Narayan6
,
Jimmy Narayan6 ![]()
1Centre for Multidisciplinary Research, Anurag University, Hyderabad, Telangana. India.
2Chitkara Centre for Research and Development, Chitkara University, Himachal Pradesh. India.
3Centre of Research Impact and Outcome, Chitkara University, Rajpura, Punjab. India.
4Noida International University, Department of Obstetrics and Gynaecology, Greater Noida, Uttar Pradesh. India.
5ATLAS SkillTech University, Department of uGDX, Mumbai, Maharashtra. India.
6IMS and SUM Hospital, Siksha ‘O’ Anusandhan (Deemed to be University), Department of Gastroenterology, Bhubaneswar, Odisha. India.
Cite as: Raju D, Mishra A, Thakur R, Sarkar A, Kolaventi SS, Narayan J. Assessment of the Risk Factors for Gastrointestinal Bleeding Among Individuals with Left Ventricular Support Machines. Seminars in Medical Writing and Education. 2024; 3:528. https://doi.org/10.56294/mw2024528
Corresponding author: Dheeravath Raju *
ABSTRACT
Gastrointestinal bleeding is a known complication among individuals with left ventricular support machines, also known as left ventricular assist devices (LVADs). LVADs are mechanical pumps that are used to support the pumping function of the heart in individuals with severe heart failure. To shield patients from thromboembolic intricacies, require anticoagulation (AC) with warfarin and insect platelet treatment. AC or anti-platelet medication is frequently reduced or discontinued in these individuals to address gastrointestinal bleeding (GI), a serious side effect. The likelihood of thromboembolic (TE) events later in LVAD therapy could be affected by changes to AC that are made to control GI bleeding. Provided continuous-flow LVADs to 389 patients, including 308 men, the typical age at implant was 60 years of age. The results were looked at to see if there was a link between GI bleeding and later TEs, which were either suspected or confirmed to be hemolytic, stroke, transient ischemic attack, or pump thrombosis. Using time-dependent variables, the relationships between GI bleeding, subsequent TEs, and mortality were investigated. There were 199 GI draining occasions in 116 of 389 patients, or 0,45 GI drains each quiet year of care. 97 of the 389 patients experienced 128 TE events, or 25 % of the total, for a rate of 0,31 TE events per patient-year of assistance. This suggests that a decrease in anticoagulation and anti-platelet therapy for the treatment of GI bleeds could increase this risk, despite the fact that the exact cause of this association is unknown.
Keywords: Gastrointestinal Bleeding; Thromboembolic; Anti-Coagulation; Co-Morbidities, Left Ventricular Assist Devices.
RESUMEN
La hemorragia gastrointestinal es una complicación conocida entre las personas con máquinas de asistencia ventricular izquierda, también conocidas como dispositivos de asistencia ventricular izquierda (DAVI). Los DAVI son bombas mecánicas que se utilizan para apoyar la función de bombeo del corazón en individuos con insuficiencia cardíaca grave. Para proteger a los pacientes de los intríngulis tromboembólicos, requieren anticoagulación (AC) con warfarina y tratamiento antiagregante plaquetario. La AC o la medicación antiagregante plaquetaria se reduce o interrumpe con frecuencia en estos individuos para hacer frente a las hemorragias gastrointestinales (GI), un efecto secundario grave. La probabilidad de que se produzcan episodios tromboembólicos (TE) más adelante en el tratamiento con DAVI podría verse afectada por los cambios de AC que se realizan para controlar la hemorragia GI. Proporcionaron DAVI de flujo continuo a 389 pacientes, 308 de ellos varones; la edad típica en el momento del implante era de 60 años. Se analizaron los resultados para ver si existía una relación entre la hemorragia digestiva y los TE posteriores, que se sospechaba o se confirmó que eran hemolíticos, ictus, accidente isquémico transitorio o trombosis de la bomba. Utilizando variables dependientes del tiempo, se investigaron las relaciones entre hemorragia digestiva, TE posteriores y mortalidad. Hubo 199 ocasiones de drenaje GI en 116 de 389 pacientes, o 0,45 drenajes GI cada año tranquilo de atención. 97 de los 389 pacientes experimentaron 128 eventos de TE, o el 25 % del total, para una tasa de 0,31 eventos de TE por paciente-año de asistencia. Esto sugiere que una disminución del tratamiento anticoagulante y antiagregante plaquetario para el tratamiento de las hemorragias digestivas podría aumentar este riesgo, a pesar de que se desconoce la causa exacta de esta asociación.
Palabras clave: Hemorragia Gastrointestinal; Tromboembólica; Anticoagulación; Comorbilidades, Dispositivos de Asistencia Ventricular Izquierda.
INTRODUCTION
Assessment of the risk factors for any given ailment or result necessitates a thorough assessment of all relevant variables that have been discovered via academic research and clinical practice. Despite the fact that could provide broad advice, it’s crucial to speak with a healthcare provider for a complete and personalized evaluation of risk factors based on the particular condition of concern. Obtain the person’s complete medical history, which should include any past diagnoses, treatments, and information on any pre-existing illnesses.(1) Examine lifestyle elements that could affect risk, such as smoking, drinking, nutrition, levels of physical activity, and environmental exposure. Assessing these variables could reveal risk factors that can be changed and then addressed with treatments.(2) Investigate the person’s family history since certain diseases could have a hereditary component. Finding hereditary risk factors could be aided by evaluating the existence of family instances or genetic predispositions.(3) Consider demographic parameters including age, gender, race/ethnicity, and socioeconomic position since they could have an impact on a person’s risk for developing an illness or experiencing a certain result(4) Behavioral and psychological aspects consider stress, mental health issues, coping techniques, and compliance with medical advice as behavioral and psychological factors that could increase the risk.(5) Biomarkers and diagnostic tests take considered the use of biomarkers and diagnostic tests that might aid in risk assessment. Laboratory tests, genetic tests, imaging investigations, and specialized evaluations pertaining to the disease of interest might all fall under this category.(6) Corticosteroids, selective serotonin reuptake inhibitors (SSRIs), non-steroidal anti-inflammatory drugs (NSAIDs), anticoagulants, ant platelet agents, and others raise the risk of gastrointestinal bleeding. It’s critical to evaluate the usage and dose of these drugs while assessing risk.(7) An increased risk of peptic ulcers and consequent gastrointestinal bleeding is linked to the bacterium Helicobacter pylori infection. Finding a possible risk factor could be assisted by evaluating the existence of H. pylori infection.(8) Misuse of alcohol and other drugs: Using drugs that irritate the gastrointestinal mucosa or abusing alcohol in excess could both increase the risk of gastrointestinal bleeding. Finding other risk variables requires evaluating past alcohol and drug misuse.(9) The gastrointestinal dying, peptic ulcers, gastro esophageal reflux illness (GERD), fiery gut sickness (IBD), or other gastrointestinal infections are significant gamble factors. For the purpose of determining the risk, the history of these circumstances must be considered.(10) Due to variables including a greater incidence of co morbidities, pharmaceutical usage, and physiological changes in the gastrointestinal tract, advanced age is linked to an increased risk of gastrointestinal bleeding.(11) Chronic medical disorders some chronic illnesses, such as renal disease, cardiovascular disease, coagulopathies, and liver disease, might make people more prone to gastrointestinal bleeding. The risk must be evaluated by determining if these circumstances exist and how severe they are.(12) An elevated risk could be attributed to a family history of gastrointestinal bleeding or associated illnesses, such as hereditary hemorrhagic telangiectasia (HHT) or inherited clotting abnormalities. Finding possible genetic predispositions could benefit from a family history analysis.(13) Smoking has been linked to a higher incidence of digestive bleeding, especially peptic ulcers. Utilization of No steroidal Anti-Inflammatory Drugs (NSAIDs) Consistent or prolonged use of NSAIDs like aspirin or ibuprofen could increase the risk of gastrointestinal bleeding.(14) Many people with LVADs need anticoagulation treatment to stop blood clots from developing within the device. This treatment raises the risk of gastrointestinal bleeding; especially if the anticoagulant medication is not given properly(15) LVAD users often have several co morbidities, such as chronic renal disease or liver failure, which might raise the risk of gastrointestinal bleeding. Individuals who have a history of gastrointestinal bleeding are more likely to have further bleeding incidents. Device-related complications: Infections pump thrombosis, hemolytic, and other issues with the device could raise the risk of gastrointestinal bleeding.(16)
Risk of gastrointestinal bleeding could be impacted by pump speed. High pump rates could raise the risk of bleeding, while low pump speeds can raise the chance of device clot development. The kind of LVAD being utilized could have an impact on the risk of gastrointestinal bleeding. A greater risk of bleeding has been reported for certain LVADs compared to others.(17) It’s critical to evaluate these risk variables in order to identify LVAD users who could be more susceptible to gastrointestinal bleeding. Cardiologists, gastroenterologists, and other medical professionals must work together in a multidisciplinary manner to effectively treat gastrointestinal bleeding in this group.(18) Problems caused by the device, People with LVADs are susceptible to problems caused by the device, including pump thrombosis, infection, bleeding, and mechanical issues. These issues could be a factor in gastrointestinal bleeding. Anticoagulation medication is necessary for many LVAD users to stop blood clots from developing within the device. Anticoagulation might expand the gamble of dying, particularly gastrointestinal dying; regardless of whether staying away from thrombosis is vital.(19) LVADs change the cardiovascular system’s hemodynamic and blood flow. These modifications could alter blood flow to the digestive system and perhaps raise the danger of gastrointestinal bleeding. In addition to being often prevalent in LVAD users, co morbidities such chronic renal disease, liver disease, or digestive problems could raise the risk of gastrointestinal bleeding. The management of patients with LVADs necessitates a multidisciplinary approach combining cardiologists, cardiothoracic surgeons, gastroenterologists, and other medical specialists. Through this cooperative approach to treatment, possible risks of gastrointestinal bleeding are thoroughly assessed, tracked, and managed.(20) All of the current LVADs are pumps with continuous flow. Figure 1 depicts the components of LVAD system.
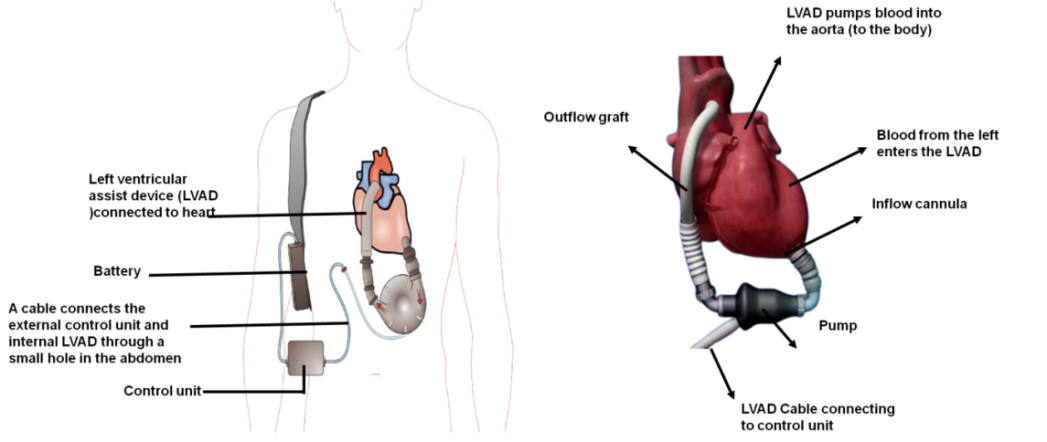
Figure 1. Parts of a system for a left ventricular assist device
The suggested connections between several path physiological phenomena that are believed to be producing GIB and angiodysplasias in individuals receiving CF-LVAD support.(21) It has been hypothesized that the onset of gastrointestinal bleeding, hemolytic pump thrombosis, and strokes are all indications of a physiological state that is abnormally hemocompatible. In this matched case-control research (1:2), 41 COVID-19 patients (31 upper and 10 lower) had GIB, but 82 COVID-19 patients’ matched controls did not have GIB. Discussion of the causes of bleeding was primary objective; the secondary objective was to discuss the outcomes and treatment options.(22) Utilizing logistic regression analysis, the odds of BTT patients with LVAD dying within a year were compared to those receiving medical treatment. In this article, the pathophysiology of hematopoietic conditions is discussed, along with their clinical manifestations and current therapies. These conditions include bleeding, acquired von Willebrand disease, heparin-induced thrombocytopenia, hemolytic, stroke, and pump thrombosis. It provides a practical clinical evaluation while summarizing the data that is available for managing these entities. (23) Calculations of the risk of severe LGIB were made using both univariate and multivariate logistic regression. To decide whether thrombolytic treatment was fruitful, incorporated all patients who had gotten at least two medicines for intermittent HVAD apoplexy. The effectiveness of thrombolytic treatment was determined to be the absence of stroke, mortality, new HVAD apoplexy, or careful gadget trade in the span of 30 days of the rate. Additional research results include mortality and heart transplantation.
METHOD
Research population
Table 1 presents an overview of key pre-existing comorbidities in patients before LVAD implantation that are critical for understanding their risk profiles and treatment needs. Such comorbidities as dyslipidemia, atrial flutter, and coronary artery disease indicate severe cardiovascular dysfunction and predispose individuals toward gastrointestinal (GI) bleeding and thromboembolic (TE) events. Complications of the vascular type are further enhanced by conditions such as chronic hypertension and diabetes, further circumventing any consideration of coagulation or bleeding tendency. A history of stroke demonstrates how the use of anticoagulation therapy attempts to balance clot avoidance alongside bleeding threats. Patients requiring long-term LVAD support often have higher rates of complications, and therefore, the anticoagulation strategies must be carefully monitored. This table offers direct suggestions towards the research by mentioning the participant factors with a role in GI bleeding incidence, TE event occurrence, and mortality trends. Appreciating those situations allows optimizing the anticoagulation management, avoiding adverse events, and improving overall outcomes in LVAD patients.
|
Table 1. Clinical Characteristics before LVAD Implantation |
|
|
Variable |
Patients [n (%)] |
|
Dyslipidemia |
232 (60 %) |
|
Atrial Flutter |
95 (25 %) |
|
Type 2 Diabetes Mellitus |
113 (29 %) |
|
History of Stroke |
49 (13 %) |
|
Coronary Artery Disease |
186 (48 %) |
|
Chronic Hypertension |
148 (38 %) |
|
Long-Term LVAD Support |
137 (35 %) |
Outcome evaluation
A case of GI bleeding necessitating hospital admission, a blood transfusion, or endoscopic or interventional radiologic care was referred to as a “GI bleeding episode.” A single occurrence was recorded when several bleeding incidents occurred over the same hospital stay. TE incidents and GI hemorrhage that happened after the LVAD was removed were not included. Within 30 days of the LVAD’s installation, TE occurrences were deemed to be connected the surgery and were not incorporated in calculation.
Statistical analysis
Demographic information and other patient-related data were gathered by utilizing the prospectively gathered clinical databases. The Student’s pairing t-test or the chi-square test was used to compare data between the two groups for continuous or categorical variables. After the Kaplan-Meier method was used to determine overall survival and independence from an outcome event, log rank was used to compare the results. The influence of GI bleeding on later TE episodes, survival, and other outcomes was examined as a time-dependent covariate. The statistic of statistical significance was P < 0,05. A patient’s death within 30 days of surgery or while receiving care at the reference hospital was considered early surgical mortality.
RESULTS
All early victims (n = 365) received data, with a median follow-up of ten months. 116 patients (30 %; 116 of 389) had 119 GI bleeding incidents, 67 (58 %; 67 of 116) of which occurred just once, and 49 (42 %; 49 of 116) of which occurred several times. The average quantity of time (from 1 to 116 months) between the LVAD implant and the first GI bleed was 5. In these individuals, there were 0,45 GI bleeds per supported patient year. Figure 2 depicts the cohort’s overall independence from GI hemorrhage.
Table 2 highlights the incidence of gastrointestinal bleeding and thromboembolic events among patients with LVAD. It illustrates how changes in anticoagulation therapy affect bleeding and clotting risks. The data show the usual complications that patients face, stressing the need for careful observation and individualized treatment approaches. Understanding the frequency of these events helps clinicians determine how to balance bleeding prevention with clot risk management. This table is directly relevant to the research because it shows how variations in anticoagulation impact outcomes for patients, thus supporting the need for individualized approaches to minimize the risk of complications while ensuring effective LVAD functioning.
|
Table 2. Incidence of GI Bleeding and Thromboembolic Events |
|||
|
Event Type |
Event Rate (/pt-year) |
Patients (n) |
Total Events (n) |
|
Upper GI Bleeding |
0,38 |
72 |
79 |
|
Lower GI Bleeding |
0,27 |
58 |
64 |
|
Suspected/Confirmed Pump Thrombosis |
0,09 |
41 |
43 |
|
Hemolysis |
0,04 |
38 |
40 |
|
Ischemic Stroke |
0,15 |
34 |
37 |
|
Transient Ischemic Attack (TIA) |
0,08 |
19 |
21 |
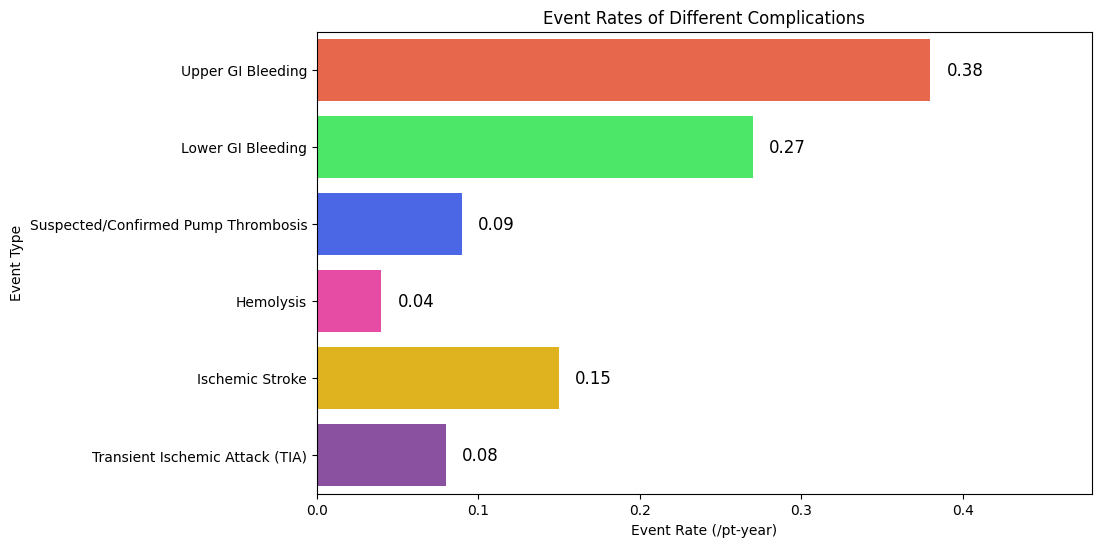
Figure 2. Independence from GI bleeding incidents throughout time
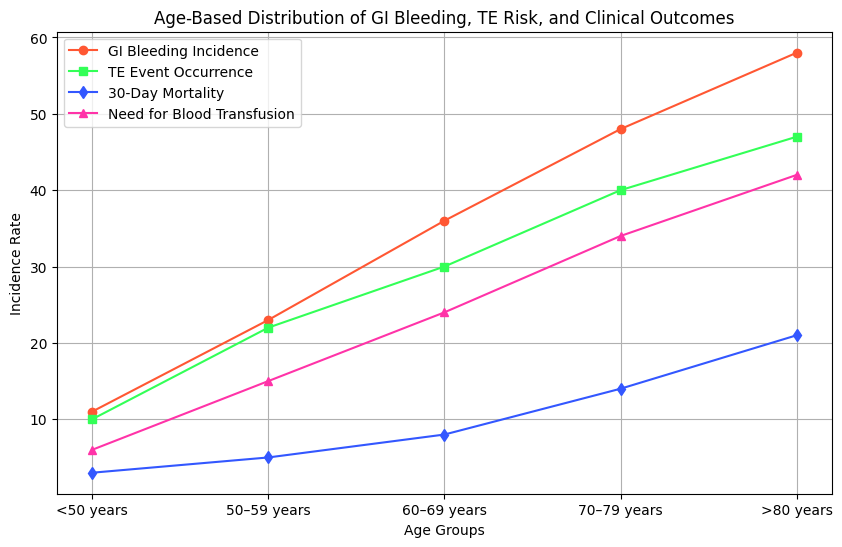
Figure 3. The absence of TE occurrences due to time
Table 3 is an analysis of the co-relation of patient’s age with the odds of experiencing gastrointestinal bleeding, thromboembolic events, and complications. Older adults have a significantly raised risk of bleeding and clotting problems, which can hence develop a hypothesis for the formulation of age-appropriate treatment strategies. Besides, the table discerns trends in the outcomes of care, inclusive of mortality and necessity of other interventions. This will support the focus in this research on optimization of anticoagulation management by showing that age-based individualized approaches can help reduce risks and enhance overall patient care. The findings emphasize the need for continuous monitoring and adjustments in LVAD therapy. Overall cohort survival is shown in figure 4. When they were looked at as time-dependent factors, the incidence of a GI bleed and a TE episode had no negative effects on late survival (HR =14 0,72 [95 % CI 0,4 to 1,2], p 14 0,1 and (HR 14 0,84 [0,5 to 1,3], p = 14 0,43) respectively.
|
Table 3. Age-Based Distribution of GI Bleeding, Thromboembolic Risk, and Clinical Outcomes |
|||||
|
Variable |
<50 years |
50–59 years |
60–69 years |
70–79 years |
>80 years |
|
GI Bleeding Incidence |
11 |
23 |
36 |
48 |
58 |
|
TE Event Occurrence |
10 |
22 |
30 |
40 |
47 |
|
Relative Risk of GI Bleed Leading to TE Event |
4,9 [1,0–24] |
6,2 [2,1–11] |
8,0 [3,4–16] |
10,5 [5,3–23] |
14,1 [6,5–32] |
|
p-value |
0,071 |
0,022 |
<0,001 |
<0,001 |
<0,001 |
|
30-Day Mortality |
3 |
5 |
8 |
14 |
21 |
|
Need for Blood Transfusion |
6 |
15 |
24 |
34 |
42 |
Figure 4. Worldwide late survival due to time
DISCUSSION
Research on the relationship between gastrointestinal bleeding and thromboembolic events in LVAD patients was conducted on one of the largest possible groups. After LVAD implantation, 30 % of patients had gastrointestinal bleeding, which was a frequent occurrence. It was discovered that GI bleeding in these same individuals was linked to a rise in the frequency of thromboembolic events that followed.(24) If the patient had experienced a past GI bleed, the incidence of thromboembolic events rose by a factor of 7,4 overall. The rise was approximately 15 times greater in patients who were 470 years old, which made the result much more striking. An LVAD support complication that is well-known is gastrointestinal hemorrhage. According to Kushner et al., 19 % of LVAD patients had GI hemorrhage at least once. The rate was somewhat greater in research, at 30 %. The percentage in the research was a little higher, at 30 %. In line with other research, it was discovered that age is the most crucial factor in determining whether a patient would develop GI bleeding.(25) Metalloproteinase can break ADAM-13 from vWF in the blood due to a change in the protein’s structure caused by elevated shear stress. High-molecular-weight vWF concentrations are reduced as a result of this destruction, which results in coagulopathy and bleeding. Aortic stenosis, hypertrophic cardiomyopathy, and inborn heart anomalies are instances of problems that increment the sheer weight on blood and have been connected to gain von Willebrand infection. It has also been seen in LVAD patients, and this decline in high-molecular-weight vWF is probably a significant contributor to the pathophysiology of GI bleeding.(26) The increased occurrence of gastrointestinal angiodysplasia in LVAD patients has been suggested as a second possible explanation for GI bleeding. Arteriovenous malformations were reportedly the cause of 31 % of GI bleeding in LVAD patients. According to neurovascular etiology presented, increasing sympathetic tone results in smooth muscle relaxation, dilated arterioles, and a rise in aortic valve substitutions. Additionally, the non-pulsatile LVADs’ narrow pulse pressure could lead to arterial dilatation and hypo perfusion of the gastrointestinal system, which heightens the development of AVMs. Even with anticoagulation medication, thromboembolic events constitute a serious side effect of LVAD treatment. A stroke, TIA, hemolytic, or pump thrombosis/power surge was all considered thromboembolic events in the research, which included 25 % of patients receiving LVAD treatment. Patient had previously had GI bleeding; these thromboembolic episodes were much inclined to occur.(27) Blood becomes hypercoagulable when it comes into direct touch with the foreign material of an LVAD, which makes platelet activation more likely. LVAD coagulopathy is caused by certain cells adhering to the device’s substrate. When providing warfarin anticoagulant medicine to patients, INR goals of 2,0 to 3,0 are often employed; however, therapy is typically discontinued if a GI bleed develops. According to the observations, the patients are more at risk for a future thrombotic episode after anticoagulation is stopped. Patients are in many cases managed warfarin anticoagulation treatment with INR focuses somewhere in the range of 2,0 and 3,0 notwithstanding subterranean insect platelet prescription; however some slight variances do occur due to disparities in philosophy and practices among organizations. Some of these slight variations relate to how anticoagulation is stopped after a bout of GI bleeding, such as: (1) aggressive INR reversal at admission for GI bleeding as opposed to monitoring and straightforward anticoagulation withdrawal; (2) choose between continuing to take aspirin or a temporary substitute such a glycoprotein IIb/IIIa inhibitor over stopping ant platelet drugs; (3) After treating GI bleeding, when should anticoagulation be restarted.(28) The bulk of people’s proposals seem to be rather similar, notwithstanding these variances in a tiny number of instances. In a clinic, that use less aggressive anticoagulation for elderly patients, who are more likely to experience GI bleeding, and more aggressive anticoagulation for younger patients, who are less likely to experience GI bleeding. Itcontends that the alteration in anticoagulation following GI hemorrhage is the primary cause of this. Even though it is common knowledge that unfavorable occurrences like GI bleeding and thromboembolic events could result in prolonged hospital stays and issues related to the hospital, there was no significant reduction in mortality as a result of these adverse events.(29) Patients utilizing the Heart Mate II LVAD were found to have the highest incidence of bleeding events when researchers looked at the hazards of thrombosis and bleeding in those patients. Patients with INRs > 2,5 the highest probability of bleeding events, INR target range of 1,5 to 2,5 was suggested by this research to reduce the frequency of bleeding episodes. According to the research, halting a bleeding could ultimately make thromboembolic problems less common.(30) The rates of bleeding events and, paradoxically, TE events could benefit by changing the initial INR targets to reduce bleeding. This absolute requirement, obviously, be weighed against the possible raised chance of thromboembolic occasions connected to decrease INR.
Limitations
These are certain limitations to this research. 389 patients at 2 centers were the only ones whose data were used. The analysis was carried out after the event, even though the data were collected prospectively. The many anticoagulant medicine adjustments made for people with GI bleeding provide a considerable obstacle to the ongoing monitoring of these patients. The patients were handled by several doctors using non-standardized methods while being seen at 2 separate locations. In the event of a GI bleed, the dosage of warfarin and ant platelet medication was not always clear. Indeed, even with various regimens, a huge association between GI drains and thromboembolic occasions was found. In LVAD patients, found a statistically significant correlation between episodes of GI bleeding and subsequent thromboembolic episode. Contend that anticoagulant medication adjustments made in reaction to the bleeding incidence have had a substantial impact on this. Controlling GI bleeding could improve outcomes for LVAD patients by reducing thromboembolic events.
CONCLUSION
In conclusion, those who have LVAD or other forms of left ventricular support machinery are more inclined to have gastrointestinal bleeding. There are many risk factors that have been discovered in this group, such as the use of anticoagulant and ant platelet drugs, the existence of co morbidities such chronic renal disease or liver failure, and the presence of a history of gastrointestinal bleeding. Furthermore, elements including device-related issues pump speed, and device type could potentially raise the possibility of gastrointestinal bleeding. To effectively control gastrointestinal bleeding in patients who are using left ventricular assist devices, physicians such as cardiologists, gastroenterologists, and others need to collaborate with one another. Thorough drug administration, keeping an eye on anticoagulant levels, and routine evaluation of gastrointestinal symptoms are all methods to reduce the risk of gastrointestinal bleeding. To fully comprehend the underlying causes and the best therapeutic approaches for gastrointestinal bleeding in this group, further research is required. This will work with the advancement of centered treatments to decrease the recurrence and seriousness of gastrointestinal draining in individuals utilizing left ventricular help gadgets, consequently working on quiet results and personal satisfaction.
REFERENCES
1. Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Berglundh T, Sculean A, Tonetti MS, EFP Workshop Participants and Methodological Consultants, Merete Aass A, Aimetti M. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. Journal of clinical periodontology. 2020 Jul;47:4-60. https://doi.org/10.1111/jcpe.13290
2. Zhu N, Yu C, Guo Y, Bian Z, Han Y, Yang L, Chen Y, Du H, Li H, Liu F, Chen J. Adherence to a healthy lifestyle and all-cause and cause-specific mortality in Chinese adults: a 10-year prospective research of 0.5 million people. International Journal of Behavioral Nutrition and Physical Activity. 2019 Dec;16:1-3. https://doi.org/10.1186/s12966-019-0860-z
3. Cannon-Albright LA, Foster NL, Schliep K, Farnham JM, Teerlink CC, Kaddas H, Tschanz J, Corcoran C, Kauwe JS. Relative risk for Alzheimer disease based on complete family history. Neurology. 2019 Apr 9;92(15):e1745-53. https://doi.org/10.1212/WNL.0000000000007231
4. NeblettJr EW. Racism and health: Challenges and future directions in behavioral and psychological research. Cultural Diversity & Ethnic Minority Psychology. 2019 Jan;25(1):12. https://psycnet.apa.org/doi/10.1037/cdp0000253
5. Hermann P, Appleby B, Brandel JP, Caughey B, Collins S, Geschwind MD, Green A, Haïk S, Kovacs GG, Ladogana A, Llorens F. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. The Lancet Neurology. 2021 Mar 1;20(3):235-46. https://doi.org/10.1016/S1474-4422(20)30477-4
6. Olsen AM, McGettigan P, Gerds TA, Fosbøl EL, Olesen JB, Sindet-Pedersen C, Staerk L, Lock Hansen M, Pallisgaard JL, Køber L, Torp-Pedersen C. Risk of gastrointestinal bleeding associated with oral anticoagulation and non-steroidal anti-inflammatory drugs in patients with atrial fibrillation: a nationwide research. European Heart Journal-Cardiovascular Pharmacotherapy. 2020 Sep;6(5):292-300. https://doi.org/10.1093/ehjcvp/pvz069
7. Patel P, Sengupta N. PPIs and beyond: A framework for managing anticoagulation-related gastrointestinal bleeding in the era of COVID-19. Digestive diseases and sciences. 2020 Aug;65:2181-6. https://doi.org/10.1007/s10620-020-06408-x
8. Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol‐associated liver diseases: 2019 practice guidance from the American Association for the Research of Liver Diseases. Hepatology. 2020 Jan;71(1):306-33.DOI: https://doi.org/10.7759/cureus.7644
9. Hawks MK, Svarverud JE. Acute lower gastrointestinal bleeding: evaluation and management. American family physician. 2020 Feb 15;101(4):206-12.DOI: https://doi.org/10.7759/cureus.7644
10.Forns J, Pottegård A, Reinders T, Poblador-Plou B, Morros R, Brandt L, Cainzos-Achirica M, Hellfritzsch M, Schink T, Prados-Torres A, Giner-Soriano M. Antidepressant use in Denmark, Germany, Spain, and Sweden between 2009 and 2014: Incidence and comorbidities of antidepressant initiators. Journal of affective disorders. 2019 Apr 15;249:242-52. https://doi.org/10.1016/j.jad.2019.02.010
11.Gimeno-Miguel A, Bliek-Bueno K, Poblador-Plou B, Carmona-Pírez J, Poncel-Falcó A, González-Rubio F, Ioakeim-Skoufa I, Pico-Soler V, Aza-Pascual-Salcedo M, Prados-Torres A, Gimeno-Feliu LA. Chronic diseases associated with increased likelihood of hospitalization and mortality in 68,913 COVID-19 confirmed cases in Spain: A population-based cohort research. PloS one. 2021 Nov 12;16(11):e0259822. https://doi.org/10.1371/journal.pone.0259822
12.Hernandez GA, Lemor A, Clark D, Blumer V, Burstein D, Byrne R, Fowler R, Frischhertz B, Sandhaus E, Schlendorf K, Zalawadiya S. Heart transplantation and in‐hospital outcomes in adult congenital heart disease patients with Fontan: a decade nationwide analysis from 2004 to 2014. Journal of cardiac surgery. 2020 Mar;35(3):603-8. https://doi.org/10.1111/jocs.14430
13.Martin TA, Wan DW, Hajifathalian K, Tewani S, Shah SL, Mehta A, Kaplan A, Ghosh G, Choi AJ, Krisko TI, Fortune BE. Gastrointestinal bleeding in patients with coronavirus disease 2019: a matched case-control research. Official journal of the American College of Gastroenterology| ACG. 2020 Oct 1;115(10):1609-16.DOI: https://doi.org/10.14309/ajg.0000000000000805
14.Bouget J, Viglino D, Yvetot Q, Oger E. Major gastrointestinal bleeding and antithrombotics: Characteristics and management. World journal of gastroenterology. 2020 Sep 28;26(36):5463. https://doi.org/10.3748/wjg.v26.i36.5463
15.Triantafyllou K, Gkolfakis P, Gralnek IM, Oakland K, Manes G, Radaelli F, Awadie H, Duboc MC, Christodoulou D, Fedorov E, Guy RJ. Diagnosis and management of acute lower gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021 Aug;53(08):850-68. https://doi.org/10.1055/a-1496-8969
16. Lau LH, Sung JJ. Treatment of upper gastrointestinal bleeding in 2020: New techniques and outcomes. Digestive Endoscopy. 2021 Jan;33(1):83-94. https://doi.org/10.1111/den.13674
17. Gudu T, Jadon DR. Multidisciplinary working in the management of axial and peripheral spondyloarthritis. Therapeutic advances in musculoskeletal disease. 2020 Dec;12:1759720X20975888. https://doi.org/10.1177/1759720X20975888
18. Zinoviev R, Lippincott CK, Keller SC, Gilotra NA. In full flow: left ventricular assist device infections in the modern era. InOpen forum infectious diseases 2020 Could (Vol. 7, No. 5, p. ofaa124). US: Oxford University Press. https://doi.org/10.1093/ofid/ofaa124
19. Malone G, Abdelsayed G, Bligh F, Al Qattan F, Syed S, Varatharajullu P, Msellati A, Mwipatayi D, Azhar M, Malone A, Fatimi SH. Advancements in left ventricular assist devices to prevent pump thrombosis and blood coagulopathy. Journal of anatomy. 2023 Jan;242(1):29-49. https://doi.org/10.1111/joa.13675
20. Pugovkin AA, Markov AG, Selishchev SV, Korn L, Walter M, Leonhardt S, Bockeria LA, Bockeria OL, Telyshev DV. Advances in hemodynamic analysis in cardiovascular diseases investigation of energetic characteristics of adult and pediatric sputnik left ventricular assist devices during mock circulation support. Cardiology Research and Practice. 2019;2019(1):4593174. https://doi.org/10.1155/2019/4593174
21. Wilkins T, Wheeler B, Carpenter M. Upper gastrointestinal bleeding in adults: evaluation and management. American family physician. 2020 Mar 1;101(5):294-300. https://doi.org/10.14740/gr1115
22. Peters AE, Smith LA, Ababio P, Breathett K, McMurry TL, Kennedy JL, Abuannadi M, Bergin J, Mazimba S. Comparative analysis of established risk scores and novel hemodynamic metrics in predicting right ventricular failure in left ventricular assist device patients. Journal of cardiac failure. 2019 Aug 1;25(8):620-8. https://doi.org/10.1016/j.cardfail.2019.02.011
23. Mehra MR. The burden of haemocompatibility with left ventricular assist systems: a complex weave. European heart journal. 2019 Feb 21;40(8):673-7. https://doi.org/10.1093/eurheartj/ehx036
24. Bansal A, Uriel N, Colombo PC, Narisetty K, Long JW, Bhimaraj A, Cleveland Jr JC, Goldstein DJ, Stulak JM, Najjar SS, Lanfear DE. Effects of a fully magnetically levitated centrifugal-flow or axial-flow left ventricular assist device on von Willebrand factor: a prospective multicenter clinical trial. The Journal of Heart and Lung Transplantation. 2019 Aug 1;38(8):806-16. https://doi.org/10.1016/j.healun.2019.05.006
25. Martin TA, Wan DW, Hajifathalian K, Tewani S, Shah SL, Mehta A, Kaplan A, Ghosh G, Choi AJ, Krisko TI, Fortune BE. Gastrointestinal bleeding in patients with coronavirus disease 2019: a matched case-control research. Official journal of the American College of Gastroenterology| ACG. 2020 Oct 1;115(10):1609-16.DOI: https://doi.org/10.14309/ajg.0000000000000805
26. Truby LK, Farr MA, Garan AR, Givens R, Restaino SW, Latif F, Takayama H, Naka Y, Takeda K, Topkara VK. Impact of bridge to transplantation with continuous-flow left ventricular assist devices on posttransplantation mortality: a propensity-matched analysis of the United Network of Organ Sharing database. Circulation. 2019 Aug 6;140(6):459-69. https://doi.org/10.1161/CIRCULATIONAHA.118.036932
27. Hilal T, Mudd J, DeLoughery TG. Hemostatic complications associated with ventricular assist devices. Research and practice in thrombosis and haemostasis. 2019 Oct 1;3(4):589-98. https://doi.org/10.1002/rth2.12226
28. Tapaskar N, Jones B, Mei S, Sengupta N. Comparison of clinical prediction tools and identification of risk factors for adverse outcomes in acute lower GI bleeding. Gastrointestinal endoscopy. 2019 Could 1;89(5):1005-13. https://doi.org/10.1016/j.gie.2018.12.011
29. Coco VL, De Piero ME, Massimi G, Chiarini G, Raffa GM, Kowalewski M, Maessen J, Lorusso R. Right ventricular failure after left ventricular assist device implantation: a review of the literature. Journal of Thoracic Disease. 2021 Feb;13(2):1256. https://doi.org/10.21037/jtd-20-2228
30. Gyoten T, Morshuis M, Rojas SV, Deutsch MA, Schramm R, Gummert JF, Fox H. Identification of characteristics, risk factors, and predictors of recurrent LVAD thrombosis: conditions in HeartWare devices. Journal of Artificial Organs. 2021 Jun;24:173-81. https://doi.org/10.1007/s10047-020-01228-2
FINANCING
The authors did not receive financing for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Dheeravath Raju, Abhinav Mishra, Rahul Thakur, Avir Sarkar, Swarna Swetha Kolaventi, Jimmy Narayan.
Investigation: Dheeravath Raju, Abhinav Mishra, Rahul Thakur, Avir Sarkar, Swarna Swetha Kolaventi, Jimmy Narayan.
Methodology: Dheeravath Raju, Abhinav Mishra, Rahul Thakur, Avir Sarkar, Swarna Swetha Kolaventi, Jimmy Narayan.
Writing - original draft: Dheeravath Raju, Abhinav Mishra, Rahul Thakur, Avir Sarkar, Swarna Swetha Kolaventi, Jimmy Narayan.
Writing - review and editing: Dheeravath Raju, Abhinav Mishra, Rahul Thakur, Avir Sarkar, Swarna Swetha Kolaventi, Jimmy Narayan.