doi: 10.56294/mw2024530
ORIGINAL
Biosynthesis of Quantum Dots: Mechanisms and Applications in Biotechnology
Biosíntesis de puntos cuánticos: mecanismos y aplicaciones en biotecnología
Prerak Sudan1 ![]() *, Tanveer Ahmad Wani2
*, Tanveer Ahmad Wani2 ![]() , Shashikant Patil3
, Shashikant Patil3 ![]() , Ambika Sahoo4
, Ambika Sahoo4 ![]() , Kollathur Sudheer5
, Kollathur Sudheer5 ![]() , B Reddy6
, B Reddy6 ![]()
1Centre of Research Impact and Outcome, Chitkara University, Rajpura, Punjab, India.
2Department of Physics, Noida International University, Greater Noida, Uttar Pradesh, India.
3Department of ISME, ATLAS SkillTech University, Mumbai, Maharashtra, India.
4Department of Biotechnology, Centre for Biotechnology, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India.
5Centre for Multidisciplinary Research, Anurag University, Hyderabad, Telangana, India.
6Chitkara Centre for Research and Development, Chitkara University, Himachal Pradesh, India.
Cite as: Sudan P, Wani TA, Patil S, Sahoo A, Sudheer K, Reddy B. Biosynthesis of Quantum Dots: Mechanisms and Applications in Biotechnology. Seminars in Medical Writing and Education. 2024; 3:530. https://doi.org/10.56294/mw2024530
Submitted: 09-11-2023 Revised: 25-02-2024 Accepted: 19-06-2024 Published: 20-06-2024
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding author: Prerak Sudan *
ABSTRACT
This article discusses the processes and applications of biosynthesized quantum dots (QD) in biotechnology. QD are nanoscale semiconductor particles with unique optical and electronic properties, making them attractive for various applications. The biosynthesis of QD involves the use of biological entities to produce nanoparticles (NPs), allowing for precise control of size and shape. QD is highly advantageous over organic fluorophores because of their distinct optical and chemical properties, and they can be utilized as fluorescent indicators for various biological applications. This article also discusses the advantages of biosynthesis over traditional chemical synthesis methods, including the use of less toxic materials and a lower environmental impact. In the future, the potential of QD is closely tied to the advancement of new generations of particles that possess precise and uniform dimensions, as well as unique optical properties.
Keywords: Quantum Dots (QD); Semiconductor Particles; Biological and Nanoparticles (NPs).
RESUMEN
Este artículo analiza los procesos y aplicaciones de los puntos cuánticos (QD) biosintetizados en biotecnología. Los QD son partículas semiconductoras a nanoescala con propiedades ópticas y electrónicas únicas, que los hacen atractivos para diversas aplicaciones. La biosíntesis de QD implica el uso de entidades biológicas para producir nanopartículas (NPs), lo que permite un control preciso del tamaño y la forma. Las QD son muy ventajosas frente a los fluoróforos orgánicos por sus propiedades ópticas y químicas distintivas, y pueden utilizarse como indicadores fluorescentes para diversas aplicaciones biológicas. En este artículo también se analizan las ventajas de la biosíntesis frente a los métodos tradicionales de síntesis química, como el uso de materiales menos tóxicos y un menor impacto medioambiental. En el futuro, el potencial de los QD está estrechamente ligado al avance de nuevas generaciones de partículas que posean dimensiones precisas y uniformes, así como propiedades ópticas únicas.
Palabras clave: Puntos Cuánticos (QD); Partículas Semiconductoras; Biológicas y Nanopartículas (Nps).
INTRODUCTION
QD are nanoscale semiconductor particles that have unique optical and electronic properties. These particles have gained significant attention in the fields of biomedicine, electronics, and energy. There are several methods to synthesize QD, but the most common one is colloidal synthesis. The biosynthesis of QD has several advantages over traditional chemical synthesis methods, including the use of less toxic materials and a lower environmental impact.(1) Additionally, biosynthesis can be performed at lower temperatures and pressures, reducing the energy required for the process.
The mechanism of biosynthesis of QD involves the use of biological entities, such as bacteria, fungi, or plants, to produce the NPs. These biological entities contain enzymes that are capable of reducing metal ions to their respective metal atoms, which can then nucleate and grow into QD. The use of biological entities allows for precise control of the size and shape of the QD, resulting in a narrower size distribution and enhanced stability. One of the major applications of biosynthesized QD is in biomedical imaging. QDs are perfect for use as fluorescent markers in biological imaging due to their unique optical features, such as their strong photoluminescence. To precisely target and label tissues or cells of interest, targeting ligands, including antibodies, can be functionalized with biosynthesized QD. Biosynthesized QD are also more desirable for in vivo imaging applications since they have been demonstrated to be biocompatible and exhibit lower toxicity than chemically synthesized QD.(2)
Another potential application of biosynthesized QD is in solar energy conversion. QD can be used as light absorbers in solar cells, where they can convert sunlight into electrical energy. Biosynthesized QD have shown promise in this application due to their narrow size distribution and enhanced stability compared to chemically synthesized QD. The fundamentals of QD, its methods of synthesis, the benefits of the biosynthetic process, its possible uses in biomedicine, its drawbacks, and its prospects for the future are covered in this study. Their study emphasized the use of QDs as FP in cancer therapy, bioimaging, DNA modification, and cell biology.
The further parts of this paper are as follows: part 2 indicates QD’s features and formation, part 3 explains the properties of Semiconductor QD Created through Biological Synthesis, part 4 discusses about current perspective and Restrictions on Semiconductor QD Use in Cell Biology, Biotechnology, and Biomedicine and part 5 shows the conclusion part.
Qd’s features and formation
Manufacturing of QDs
The basic principle behind the majority of QD preparation techniques is to put their chemical precursors together to create nanocrystals, which then become QDs (a process known as the bottom-up strategy). It can be produced using a range of chemical and physical techniques. A few approaches that show promise for producing QDs that are adaptable, reproducible, and uniform. Figure 1 displays the preparation of QDs.
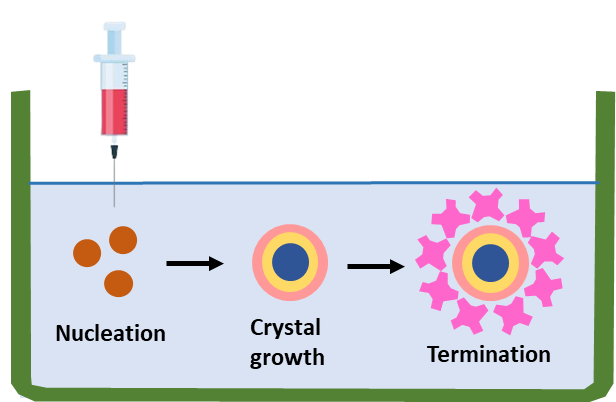
Figure 1. Manufacturing of QDs
One of the most reputable and frequently applied methods is the production of QDs by colloidal synthesis. The concept is based on turning the precursor elements into molecules by injecting them into a solvent at a high temperature. After that, the precursor molecules form nuclei (the nucleation process) and develop into nanocrystals (the crystal formation step). The physicochemical characteristics of manufactured QDs are optimized in the latter phase, where the crystallization procedure is terminated and the QDs are removed from the solvent, commonly known as organometallic synthesis.(3)
QD’s characteristics
The optical characteristics of QDs are mostly determined by their particle size. One essential method that is frequently utilized in the characterization of different Nanomedicines is DLS. However, due to the ultra-fine characteristics of QDs, more sensitive and dependable approaches are required. Particle size, shape, and internal structure QDs can all be accurately determined by the use of electron microscopy techniques such as AFM, TEM, and SEM. The QDs particle dimensions and composition can be achieved through the use of intrinsic optical features, photoluminescence, and Raman scattering spectroscopies.(4)
Basic Parameters and QD’s Optical Qualities
QDs are spherical, fluorescent semiconductor nanocrystals encased in another semiconductor material. They are made up of a semiconductor core that contains group 2-4 or group 3-5 ions. A typical QD has an atom count of around 250–10,500 and a diameter of 3–12 nm. Like semiconductor nanocrystals. The special optical, electrical, and optoelectronic capabilities of the aforementioned nanostructures have been demonstrated for a variety of uses. QD has wide intake, limited and symmetric, and slowly changing emission spectrum accelerating excited-state decay rates, all of which contribute to their great photostability. Their emission colors can be changed based on their dimensions, surface chemistry, and chemical composition, ranging from ultraviolet to visible and NIR frequencies.(5,6) It indicates that comparatively big QD exhibits low-intensity emission and a red luminosity range. More compact NPs saw green and blue fluorescence at the same time but with a higher amount of radiation strength. The quantum confinement effect controls how the optical properties of nanocrystals reflect on their diameter.
The unique flaw of QD, or so-called flashing, was discovered to be the fluctuations of their illumination over time. The brief transmission of a charged QD is what causes this occurrence, which is a stochastic event. As a result, such a loss of fluorescence can endure for a short while. QD solutions with mercaptoethanol or other reducing agents can also be used to reduce blinking by improving the nanocrystals. It is widely recognized that there are various QD classes based on their chemical makeup, arrangement, and manufacturing processes. There are suspended and epidermal QD, for instance.(7) To achieve the highest quantum yield of the nanocrystals is deposited in two mono-layers as the shell. It is feasible to create fluorescent tags with luminous maxima at any point in the optical spectrum by altering the size of the core; these labels could be used in investigations to identify various cell biology components, for visual encoding, and as part of the energy transmission system.
Chemical Approaches for QD Creation
In simple terms, there are two methods for synthesizing NPs: the bottom-up method and the top-down method, via bigger particles to guide their assembly, scientists attempt to create NPs via the top-down method. Figure 2 depicts the two approaches for generating QD. Starting at the molecular level and carefully controlling molecule form, the bottom-up method leads to bigger and more sophisticated organisms. High-temperature organometallic biosynthesis or development in nonpolar environments are the two most efficient ways to make QD chemically. The process is required during synthesis to keep the QD stable in a fluid solution.
The QD should be stable, soluble in physiologic buffers, photobleach-resistant, and have particular functions that may form conjugates with biomolecules in the ligands (thiol-containing elements). The organometallic technique offers several benefits, including the ability to regulate the QD’s development phases, the produced nanocrystals have a narrow size distribution, and even more separation and purification of QD.(8) The following stages constitute this process of chemical synthesis: Ostwald ripening stage, crystal nuclei progress, and nucleation. In this manner, luminous cadmium and selenium QD were created. Depending on the required size of the NPs, cadmium, and selenium nanocrystal nuclei were produced at temperatures over 280 ℃ for a period ranging from a few seconds to several hours. Using a high concentration of monomers and strong anisotropy of nanocrystals, technology enables you to regulate the form of NPs along with the dimension specified. The nanocrystals stretched and formed classical rods, or nanorods, due to their rapid development.(9)
A low growth rate is a necessary need for the creation of spherical NPs. By choosing settings where the nuclei’s crystalline form deviates from hexagonal, allowing crystal development in four separate instructions more complicated forms of the nanocrystals can be produced. The manufacture of QD in an aqueous phase employing stabilizers like thioglycerin and polyphosphate is another form of chemical manufacture. It was found that the selection of particular stabilizing agents affects the nanocrystals’ rate of development and charged surface. For instance, the manufacturing of NPs was effectively coated and produced significant amounts of photoluminescence by using glutathione, a substance that removes heavy metals from the cells of plants.(10)
Targeted QD deposition and UV irradiation of the QD resulted in a major improvement of the CdTe properties following further processing of the original solution. As a result, mostly bigger QD precipitate. To divide the original solution into 12–14 fractions that each included QD with an identifiable range.
The synthesis of QD in an aqueous medium is typically gradual and can take a few days to complete. While this technique was often employed in the past, alternative techniques are quickly emerging for the rapid creation of high-quality QD. The fundamental approaches for the chemical production of the biocompatible QD are therefore those that are indicated. These methods have several variations that are known.(11) Each process has certain benefits and drawbacks that are significant for the later uses of the created NP.
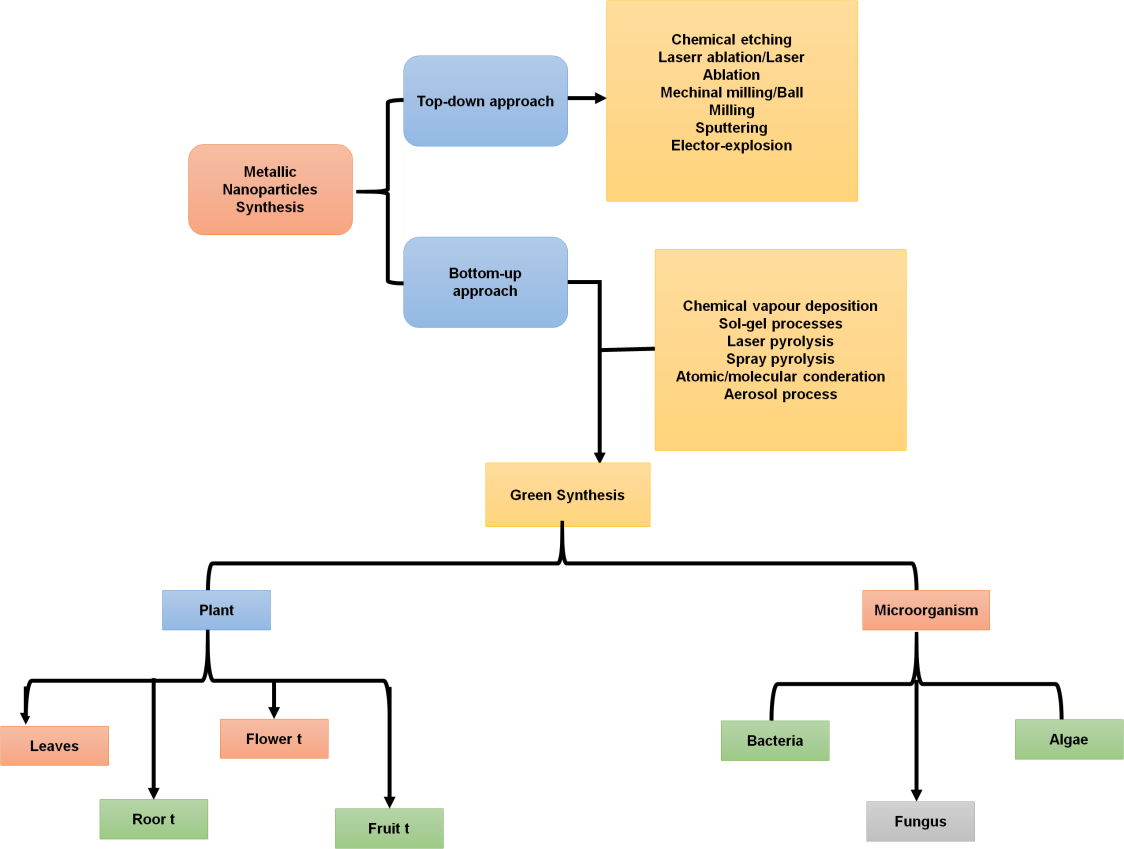
Figure 2. Two approaches for generating QD
The QD should be stable, soluble in physiologic buffers, photobleach-resistant, and have particular functions that may form conjugates with biomolecules in the ligands (thiol-containing elements). The organometallic technique offers several benefits, including the ability to regulate the QD’s development phases, the produced nanocrystals have a narrow size distribution, and even more separation and purification of QD.(8) The following stages constitute this process of chemical synthesis: Ostwald ripening stage, crystal nuclei progress, and nucleation. In this manner, luminous cadmium and selenium QD were created. Depending on the required size of the NPs, cadmium, and selenium nanocrystal nuclei were produced at temperatures over 280 ℃ for a period ranging from a few seconds to several hours. Using a high concentration of monomers and strong anisotropy of nanocrystals, technology enables you to regulate the form of NPs along with the dimension specified. The nanocrystals stretched and formed classical rods, or nanorods, due to their rapid development.(9)
A low growth rate is a necessary need for the creation of spherical NPs. By choosing settings where the nuclei’s crystalline form deviates from hexagonal, allowing crystal development in four separate instructions more complicated forms of the nanocrystals can be produced. The manufacture of QD in an aqueous phase employing stabilizers like thioglycerin and polyphosphate is another form of chemical manufacture. It was found that the selection of particular stabilizing agents affects the nanocrystals’ rate of development and charged surface. For instance, the manufacturing of NPs was effectively coated and produced significant amounts of photoluminescence by using glutathione, a substance that removes heavy metals from the cells of plants.(10)
Targeted QD deposition and UV irradiation of the QD resulted in a major improvement of the CdTe properties following further processing of the original solution. As a result, mostly bigger QD precipitate. To divide the original solution into 12–14 fractions that each included QD with an identifiable range.
The synthesis of QD in an aqueous medium is typically gradual and can take a few days to complete. While this technique was often employed in the past, alternative techniques are quickly emerging for the rapid creation of high-quality QD. The fundamental approaches for the chemical production of the biocompatible QD are therefore those that are indicated. These methods have several variations that are known.(11) Each process has certain benefits and drawbacks that are significant for the later uses of the created NP.
Physical Techniques for Synthesis of QD
Many typical physical approaches are also known, in addition to the stated chemical methods for QD production. For instance, molecule beam epitaxy is one of the various ways to deposit single crystals. When performing molecular laser epitaxy, an ultrahigh vacuum or high vacuum () is used. The main feature of this approach is the low accumulation rate, which enables epitaxial growth of the films (usually less than 3,200 nm per hour). RHEED, or reflections high-energy electron diffraction, is frequently employed in operation to track the development of the crystal levels.(12) In this particular situation, lowering the amount of strain energy is what motivates the development of QD. If the layer on the substrate is thickened, elastic stresses will develop, which will cause QD to form on the surface. As a result, numerous tens of angstrom-sized InAs pyramids surround the outermost layer. Figure 3 displays the QD synthesis physical methods and ideal properties.
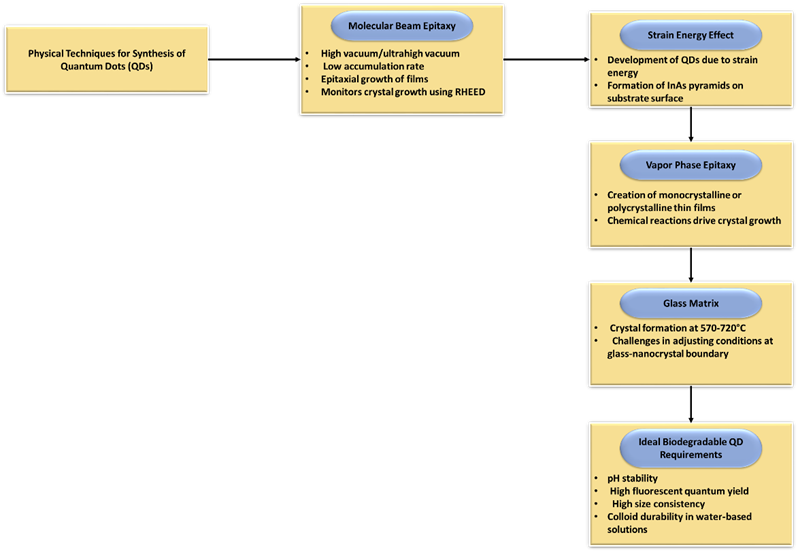
Figure 3. Physical techniques and desirable characteristics of QD synthesis
Vapor phase epitaxy is a different physical method for growing crystals. It is a technique for creating thin films that are either monocrystalline or polycrystalline. Growing crystalline layers to produce intricate semiconductor networks of layers is a very complicated operation. Crystal growth occurs by chemical reactions as opposed to physical deposition in molecular beam epitaxy. As a result, this method was selected for the creation of devices including thermodynamically MAs, and it has developed into a crucial step in the production of optics. By employing this technique, it is possible to obtain QD on the substrate as well as on and substrates.
In a glass matrix, crystal formation takes place between 570 and 720. The large variety of size ranges and difficulty of changing the conditions at the glass-nanocrystal border are drawbacks of this approach. In overall, it must be highlighted that ideal biodegradable QD requires pH stability, high fluorescent quantum yield, high size consistency, and colloid durability in water-based solutions.(13) Although it is challenging to fulfill both of these requirements at once, the extraordinary significance of QD with these characteristics encourages ongoing research.
Properties of semiconductor qd created through biological synthesis
Due to its remarkable optics, substance, photochemical, electronic, and electromagnetic capabilities, NP biogenesis is of utmost interest. Understanding the biosynthetic mechanism underlying the creation of metal NPs inside a biological system is vital for improving control over the process and its results.(14) The major reaction that takes place during the biosynthesis of NPs is decline/corrosion. Figure 4 depicts the biosynthesis of NPs. Microbial enzymes or phytochemicals from antioxidant or minimizing plants usually reduce metal compounds into NPs.
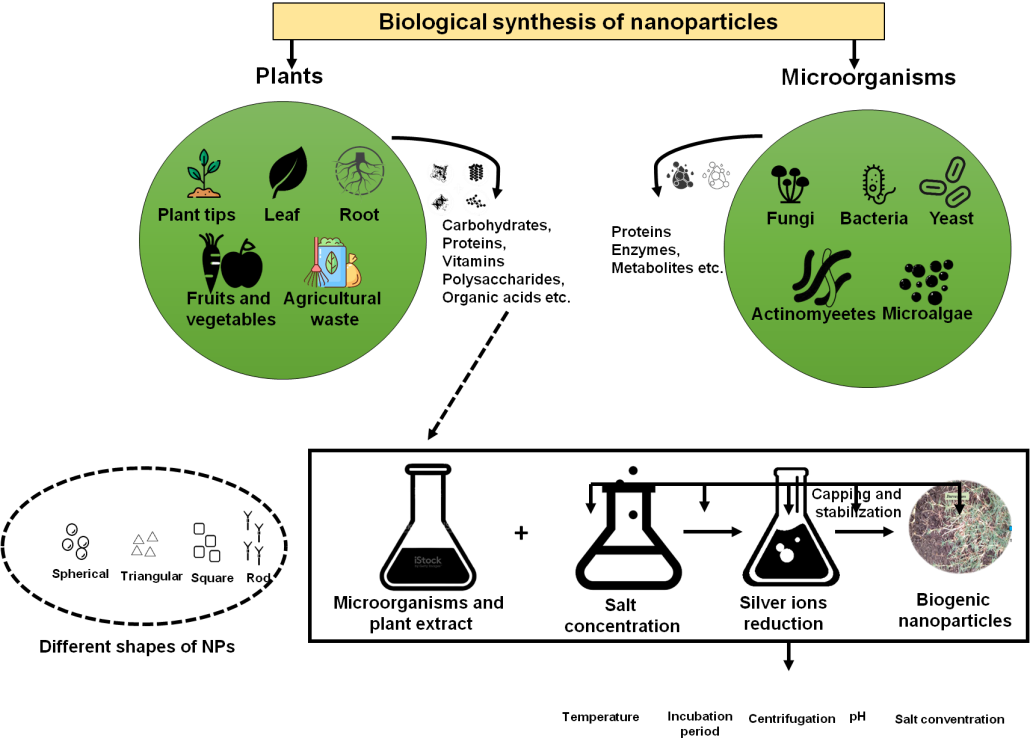
Figure 4. Biosynthesis of NP
By applying earthworms for the biosynthesis of QD
Earthworms the species rubellus can collect more cadmium than 1000 of its entire dry body weight. Although the earthworm’s cadmium detoxification mechanism is well understood, little is known about the processes of tellurium absorption, trafficking, and storage. The glutathione reductase converted the tellurite to H2Te, a common Te precursor, by reducing GSH and NADPH through the GS-Te-SG complicated. This allowed the tellurite to interact with the accessible Cd2+. The substances were transported via metallated metallothionein networks to chloragogenous tissue on worm intestines, generating QD, and isolated in earthworms exposed to CdC_2 and Na2TeO3. The particles’ absorbance band edge measurements, which correspond with CdTe QD, were 470 nm with a small excitonic signature at 380 nm.(15)
The resulting particles were useful for pertinent image analysis applications, had quantum-confined semiconductor optical characteristics, and were easy to gather. Such particles are equivalent to thiol-capped CdTe made using conventional benchtop techniques, and if this technique is expanded to additional functionally significant semiconducting materials, it might provide a handy supply of luminous probes that are consistent with living organisms.
Biosynthesis of QD Using Yeasts
To create internal Cadmium sulfide NPs, utilized the yeasts Saccharomyces pombe and Candida glabrata. The initiation and development of Cadmium sulfide crystallites to peptide-capped internal particles with a diameter of 18 are regulated by short chelating peptides having a basic structure of (). With decreasing particle diameter, manufactured Cadmium sulfide crystallites’ optically band gaps widen. Compared to manually generated Cadmium sulfide particles, these Cadmium sulfide QD proved more monodisperse.(16) These Cadmium sulfide NPs were also stable at the normal pH level and displayed a luminous peak at 470 nm.
By treating yeast cells with the inexpensive inorganic salts and in a modified “Czapek’s medium” at low humidity, the QD was created using an extracellular growth method. Following a day of incubation, the QD displays strong, constrained emission PL spectra with a modest entire width at a maximum of half, demonstrating the QD’s limited size change. By using TEM and XRD, the shape and crystalline of the bio-produced QD were evaluated. The biosynthesized QD exhibits strong luminescence, and by adjusting the particle size, it is possible to regulate the emission throughout the wavelength range of 480 to 550 nm. The protein-capped QD exhibits good biocompatibility and is evenly distributed throughout the cells, enabling straightforward real-time biological imaging of the yeast cells. By using TEM and XRD, the shape and crystallinity of the biosynthesized QD were examined in.(17) The biosynthesized QD cultured with a consistent dimension of around 3,4 nm, according to TEM images.
Biosynthesis of QD Using Diatoms
To prevent heavy metal intoxication, microbes, algae, and fungi have evolved a common detoxification strategy that includes the intracellular sequestration of metal ions using peptides similar to glutathione. The metal-triggered PC synthase uses glutathione as a substrate and produces these stimulated peptides, known as phytochelatins. PC has a recurrent pattern of pairings that result in polypeptides with the general formula, where n amounts can range from 3 to 7. They hold metallic ions in their bonds. Native Cadmium sulfide particles differed in size, sulfide concentration, and optical spectroscopy.(18) Phytochelatin 2 oligopeptide did not attach. At pH 4.9, Cadmium sulfide particles half-dissociated. P. tricornutumphytochelatin-coated Cadmium sulfide crystallites have particle size variability. This microalga generated phytochelatin-coated Cadmium sulfide nanocrystallites with radii ranging . The optic spectra of the first eluting fractional showed the biggest sulfide/Cd ratios and the lowest electronic shift energies.
P. tricornutum cells exhibit a spectrum of complexes with varying sulfide/Cd ratios, resulting in size differences due to phytochelatin-coated Cadmium sulfide crystallites, which may explain its decreased Cd sensitivity compared to other marine diatoms.
Utilizing bacteria for QD biosynthesis
A lot of interest in the biological systems-based production of nanocrystals including and . One usual form of sulfide NP produced by bacteria is the Cadmium sulfide nanocrystal. Clostridium thermoaceticum can precipitate Cadmium sulfide from on its outer layer and in the growth medium when cysteine hydrochloride is present. Cysteine most likely serves as the origin of sulphide in this situation. It has been demonstrated that when and salty sulfide are mixed, Escherichia coli can create intracellular CdS nanocrystals for the first time.(19)
The size flow of the substance called crystal phase that makes up the nanocrystals ranges from 3 to 6 nm. When compared to the late logarithmic phase, stationary phase E. coli cells produced around 25 times more nanocrystals. These findings demonstrate how several genetic and physiological factors might promote nanocrystal production within bacterial cells.(20) Cellular nanocrystals were most common during the stationary phase and were greatly influenced by the strain that was utilized as well as the cells’ development phases.
The quantity of free thiols and glutathione found in the tissues was attempted to be correlated with these findings. While these factors could have a role, no pattern appeared that accurately foretold the creation of nanocrystals. The cellular thiol level may be important, it does not affect the capacity of different E. coli strains or the development phase to generate nanocrystals. It can be possible to manipulate the generation of microbial-generated nanocrystals by understanding the physiological and genetic processes underlying nanocrystal production in Escherichia coli. For instance, inorganic synthesis uses variable ratios of many cap materials to create NPs with diverse forms, such as strings or diamonds.(21) It can be able to adjust nanocrystal crystallinity, shape, size distribution, and optical properties by manipulating E. coli’s production and quantities of polyphosphates, fatty acids, and small thiols.
The use of E. coli culture results for the effective manufacture of cadmium sulfide nanomaterials has been demonstrated. It was demonstrated that they have a maximal luminescence signal at 454 nm, which is characteristic of microorganism-produced cadmium sulfide nanoscale. For the first period, the spectral method was used to examine the stability of the produced QD. After a sample was created, NPs continued to glow for 10 days, a month, and three months.(22) Emission electron microscopy was used to show that QD has a diameter between 3 and 7 nm, an outline that is close to cylindrical, and lacks surface flaws. As a result, the prokaryotic reproductive system of E. coli is straightforward but effective and has the potential to shed light on the essential elements of nanocrystal formation in live cells. It can be able to alter the quantity, design, and crystal layout of nanocrystals produced via biosynthesis by knowing the factors involved in the process. Additionally, the most desirable materials for use in fields like rapid optical switches and infrared optical devices are NPs. Multiple methods, including colloidal aqueous and micellar solution synthesis, can be used to create NPs. Materials must be acquired under uncomplicated, hygienic settings. The production of zinc sulfide is anticipated to be clean when employed as biological probes for medicinal purposes in nanomaterials. Soluble sulfate serves as the sulfur source in the biological method used to create ZnS NPs.(23) The following explanation explains how R. sphaeroides’ biological process of transformation produces ZnS nanomaterials. Sulfate permease makes it easier for soluble sulfate to be transported to the internal membranes of the R. sphaeroides cell after first entering immobilized beads by diffuse.
Biosynthesis of QD Using Plants
The use of plant material for the creation of nanostructures is proving to be more advantageous than employing microbes because they include a wide range of enzymes that may serve as reducing agents and capping and so enhance the method of reducing and durability of nanomaterials. Specifically, scientists have revealed a unique, environmentally benign method for producing NPs quickly utilized. Particularly, the sealing and decreased properties of latex, proteins, and phytochemicals when used to make iron NPs limit aggregate formation. Potential reducers and stabilizers include the curcacycline B, a nonapeptide, and, cyclic peptides curcacycline A, an octapeptide, as well as the enzyme curcain. ZnS NPs were discovered to have a median dimension of 12 nm. Under the current testing setup, the sulfide () ions that give rise to the Zn ions are produced by the latex of J. curcas L. directly. It suggests that thiol or cysteine residues in the curcain enzyme could be the source of sulfide () ions, although their origin remains unknown.(24) It was discovered that the 0,4 % latex solutions. However, nothing is known currently regarding the biosynthesis of the semiconductor Cadmium sulphide QD with plants. For example, a new strategy for the production of cadmium sulphide QD has recently been proposed employing a Linariamaroccana L., a blooming plant, with matted roots. Transmission electron microscopy was utilized to confirm that the QDs produced are spherical and have a size range of 3 to 9 nm.
By using fungi for QD biosynthesis
The internal synthesis of Cadmium sulfide in yeast is mediated by a mechanism that involves sequestering the ions by sequences linked to cysteine with the subsequent creation of Cadmium sulfide inside the yeast’s cells, whereas enzymatic pathways in sulfate-reducing bacteria are reasonably well characterized and recognized.(25) Eukaryotic species like fungi can produce Cadmium sulfide and other metal sulfide NPs through entirely enzymatic means outside their cells. Fusarium oxysporum produces highly stable Cadmium sulfide NPs in solution when exposed to aqueous C+ and S 2+ ions. At first, the reaction solution was colorless. The presence of Cadmium sulfide NPs in the solution is indicated by the vivid yellow hue of the solution following the interaction relationship between the biomass of Fusarium oxysporum and its inorganic elements.
The UV-visible spectra of Fusarium oxysporum solution show a modest absorption edge, gradually strengthening overtime during the reaction. The quantum size regime of Cadmium sulfide ions is characterized by the existence of the absorption edge at. Cadmium sulfide NPs in a colloidal solution did not cluster after a month of storage. Absorption at 260 nm indicates protein in the reaction media. The scientists claim that the Cadmium sulfide NP solution’s long-term endurance comes from the proteins that adhere to the NPs’ surfaces and prevent aggregation.(26)
Fusarium oxysporum’s method for producing extracellular Cadmium sulfide NPs by reducing sulfate ions with enzymes was expanded to create and NPs by starting with the appropriate sulphate-containing salts. According to preliminary research, it is conceivable to create these chemical compounds via fungus-based external biotransformations. The approach described here produces stable semiconductor NPs externally, which is a major advancement over other biosynthetic approaches in how strong NPs remain enmeshed in the cell backing. The authors utilized the mycelium of the Pleurotusostreatus fungus for the effective production of Cadmium sulfide QD when cultured with and salts.
It was determined by spectral analysis of the produced particles that the detected absorption peaks are characteristic of the Cadmium sulfide nanoscale. The production of Cadmium sulfide NPs is observable by the 390 nm centroid of the intense surface plasmon resonance the presence of proteins in the interstitial broth contributes to an absorption band at 250–260 nm, which also makes a reducing enzyme-based procedure for the creation of Cadmium sulfide NPs conceivable. Even a month after the reaction, there was still no sign of flocculation of the particles in the exceptionally stable solution. By measuring the fluorescence of the Cadmium sulfide NPs and stimulating the reaction mixture at 350 nm, luminescence properties were investigated in a 460 nm-centered emissions band.
Biosynthesis of QDs
Shewanella bacteria are commonly employed in the manufacture of QDs. were produced at room temperature on air with nontoxic chemicals in a water solution, and their size ranged from 9 to 12 nm. The impact of specific variables on the size of formed by the bacterium Shewanella oneidensis in an aqueous solution with the incorporation of sodium thiosulfate and silver nitrate, as well as temperature, reagent focus, and the length of time the bacterial strains had been placed on the quantum yield, were also examined. Bacillus subtilis is another bacterium that can be used to create QDs. The primary protein that provides the synthesis phase is flagellin.(27)
Biosynthesis of QDs
The features of quantum dots, which are attractive for research, are expressed in a variety of applications, involving devices for storing energy, solar cells, photo filters, photoelectric electrodes, optoelectronic devices, and refrigerants. QDs were effectively synthesized using a variety of natural capping substances, including C. sinensis, chitosan, glucose, and ascorbic acid. The initial biomatrix that was chosen directly influenced the different structures of the generated QDs, which included spheres, nanocubes, nanorods, and sheets, with diameters ranging from 8 to 96 nm.(28)
Benefits of “Green” NP Synthesis
The “green” synthesis of nanomaterials is inexpensive, environmentally friendly, quick, and harmless is used in medicine. However, to assess the potential toxicity of these NPs. QD’s toxicity is linked to their physicochemical composition. It is impossible to fully understand all of the toxicity pathways because of the wide variety of semiconductor NPs ( and others) and capping methods () used.(29) Figure 5 depicts the process of green synthesis.
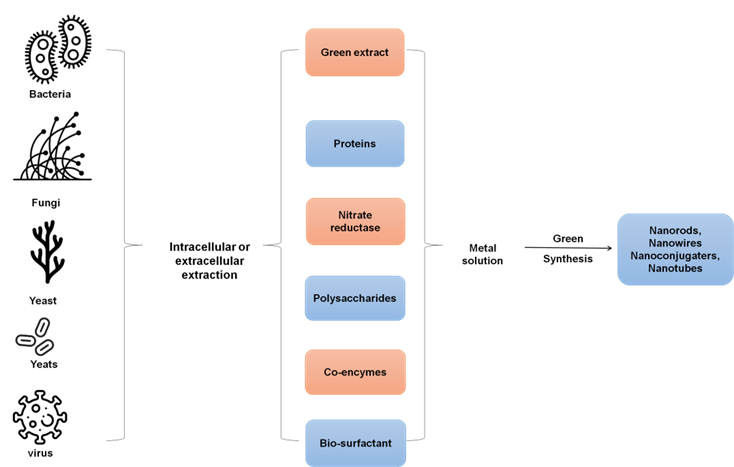
Figure 5. Process of green synthesis
By selecting the nanomaterials’ capping, using modest quantities, and changing the nanoscale size, numerous efforts were made to reduce the hazardous effects of NPs. All of these elements have a role after a cell cytotoxic reaction, QD are used as fluorescent indicators. The main causes of QD toxicity are the direct release of metal ions (such as Cd2+) or the stimulation of ROS production. These reactions have the potential to alter the structure of the majority of cells, leading to DNA damage. To maximize the utilization of QD in the target cells, various biological problems need to be resolved.(30)
Advanced quantum dots as probes for cytoskeleton mapping
Plant Genetic Conversion QD
DNA-coated NPs absorb cells without cell walls and start creating genetic material on the DNA. Besides, semiconductor NPs (QD) between 4 and 6 nm in size have been utilized as carriers to transfer chemicals into cellular. It is difficult to deliver foreign nucleic acid molecules to plants because they have cell barriers. Current approaches to plant genetic modification rely on invasive delivery. The outer layer of plant cells acts as an obstacle to the transport of exogenously supplied chemicals. The delivery of genes and small molecules into walled cells of plants has been accomplished using a variety of invasive cell delivery techniques, such as Biolistic conveyance (gene gun), microinjection, electroplating, and the conversion of a have all been used to transfer genes, but only microinjection has proven successful in delivering proteins.(31) The cell membrane of the plant’s protoplasts must be taken off before transferring nucleic acid molecules through NPs to the plant cellular. Linear nucleic acid molecules can transport specific gene sequences without adding extraneous nucleic acids, allowing for efficient transgenic organism regulation. Linear nucleic acids can PEGylate nonmaterial sometimes. Some implementations allow QD semiconductor NPs.
It developed a method to deliver a compound into plant cells by coating NP surfaces with PEG, generating PEGylated NPs coated with a specific nucleic acid molecule. These NPs are then placed in contact with the cell’s wall and nucleic acid molecules, allowing them to be taken up by small particulates. These methods also provide effective means of introducing a characteristic into a plant. The procedure involves providing plant cells, generating PEGylated NPs, coating them with plant trait expression methods, interacting with the NPs and plant cells, and allowing acceptance of the NP and the plant trait transfer technique.(32)
The study suggests that ideal plant cells or nucleic acid molecules, initially used in crosses to produce seeds, F1 hybrid cells, or desirable plants, entered the plant or cell through nanoscale absorption along cell membranes.
QD as Fluorescence Probes for Mapping the Cytoskeleton
Due to their improved fluorescence intensity and bleaching resilience, QD has generated a great deal of attention as labels for bioimaging. Direct fluorescence detection is a dependable and effective way to tag biomolecules and cellular structures using QDs. For instance, when the target molecules are coupled to streptavidin-associated QD. For instance, it was revealed that tubulin molecules are self-assembled within microtubules. QD was coupled to tubulin after being coated with inert silica shells. The assembly process was effectively visualized as a result of this.(33) Direct immunofluorescence was then performed after the fluid rearrangement of microtubules during the cell cycle using QD-labeled reagents.
The QD were coupled to monoclonal antibodies towards tubulin using two different methods: across a mineral interface (silica) and a biological contact (BSA). The small tubule in tobacco cell line individuals BY-2 were then labeled using both varieties of QD-based cooperative. The signal from NPs linked to microtubules was obtained using nanocrystals coated with BSA and coupled to antitubulin antibodies. The -produced QDs are displayed in figure 6.
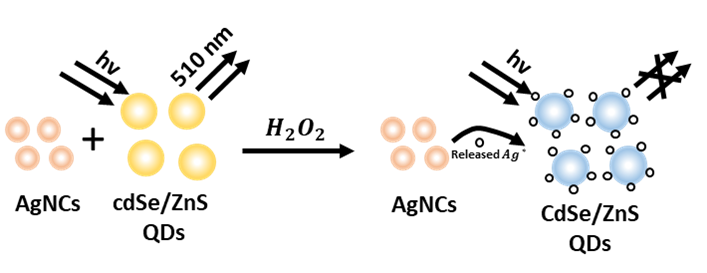
Figure 6. H_2 O_2-produced CdSe/ZnS QDs
Antitubulin antibody-conjugated silica-shelled nanocrystals were employed to provide an ongoing microtubule signal. Prematotic transitional cells’ outer perimeter displayed punctate signals when the QD-based antitubulin conjugates were used. The developing spindle was discovered to be encircled by nanocrystals once the DNA was bundled into chromosomes throughout the prophase.(34) The signal was linked to the scattered chromosome in the cell plane and reallocated to the daughter nuclei’s nuclear envelopes in the final telophase, indicating microtubular redistributions throughout the cell’s life cycle.
In situ QD fluorescence hybridization
The fact that QD is regarded as an acceptable and prospective detector for cell biology should be emphasized. Particularly, QD can be created for DNA sensing. When studying the position of each cooperative DNA molecule’s direction and position within tissues under fluorescence microscopy, QD showed a great imaging capability. In addition, the QD can be used for the detection of DNA alterations. The technique for Cadmium sulfide QD-mediated probing-based DNA spot mutation detection. QD is used in the PCR process to DNA methylation. As was already noted, QD can be effectively tailored for the detection of DNA molecules.(35) First, it emerged that QD was effectively used for FISH investigation. A thorough hybridization approach was developed using relatively tiny probes (170–700 bases) for analyzing DNA, digoxigenin-tagged. Digoxigenin-targeted antibodies were coupled with QD with maximal emission at 675 nm wavelength and employed as a detection tool. This technique is thought to be appropriate for histology section testing and chromosomal analysis. It is appropriate for one DNA locus identification per analysis, nevertheless. QD can readily endure high hybridization temperatures for telomere tagging as they have better temperature stability than organic fluorochromes. After this process, the great most fluorescent substances suffer damage and lose their fluorescent properties. A study claims that labeling was carried out utilizing QD-based combines with luminescent peaking at ranges of 610, 660, and 710 nm. The findings of traditional FISH and Southern blot assays and those obtained with QD were comparable. It would seem that the ability to combine immunophenotyping with telomere length-defining analyses is an advantage of using QD in this situation. Figure 7 displays the magnetic and fluorescent properties of QDs-antibodies.
It’s crucial to remember that in the interface of the QD surface and the integrating sequencing of DNA, the existence of the appropriate intervening components is necessary for an efficient probe-to-nucleic acid interaction. Additionally, fluorescence hybridization utilizing QD has drawbacks, most significant excessive noise and uneven staining when probes are utilized for whole-chromosome analysis.(36) It should be stressed that the high strength of the QD signal allows for the application of fragile DNA probes in QD to identify certain genes by hybridization. There are currently QD-streptavidin-based probes created to detect mRNA in tissue slices.
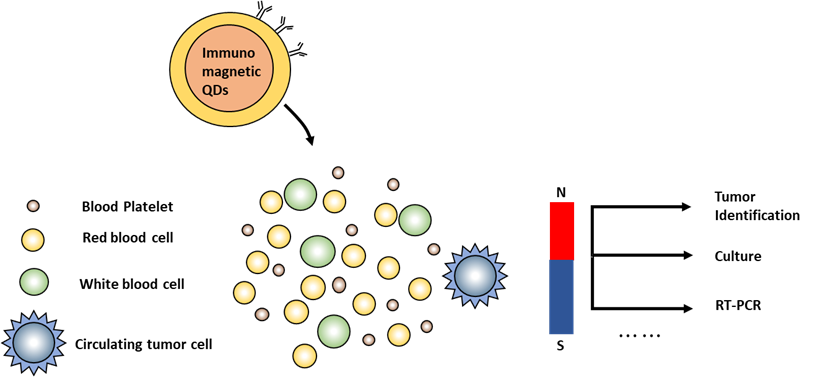
Figure 7. QDs-antibody with magnetic and fluorescence characteristics
QD as Assessing Intracellular Molecules Labels
Cell biology focuses on understanding signal transmission and response to external environment changes. Membrane-bound receptors interact with external ligands like growth factors, lipoproteins, and transferrins, which are then endocytosed and transported to endosomal compartments. To comprehend the way cells’ receptor-mediated endocytic activities are regulated and tissues, novel QD-based biological imaging approaches must be developed. Fluorescence microscopy has been utilized to follow several cooperative-mediated endocytic trafficking processes in live cells because of QD’s luminosity and photostability.(37) Labeled conjugates can enter cells by attaching to proteins on the cell membrane surface or by using the endocytosis route. It uses QD-EFG conjugates to monitor EGF receptor dimerization and signal transduction, detecting initial phases of the TK-dependent signal pathway in cells, revealing QD’s high affinity for EGF receptor erbB1. It is believed that bonding process will aid in our knowledge of the mechanisms behind intracellular retrograde transport. The actual time endocytic behavior of NGF receptors has been observed using QD coupled to NGF at a nanoscale sensitivity.
Significantly, QD works admirably to identify endings for an extended period without causing any harm. To understand the kinetics of how acetylcholine receptor clusters assemble in the postsynaptic membrane, biotinylated-bungarotoxin was coupled to streptavidin-conjugated QD. QD are thus potential tracking agents to the dynamics of membrane receptor surfaces. QD is utilized in investigations on living cells for both generic cell labeling and targeted labeling of certain cell structures.(38) Based on the experimental findings, QD designed to engage internal transportation proteins is effective at following molecules in fetal blood cells and human bone marrow. It has been demonstrated that QD effectively labels cells during proliferation and that signals originating from QD may still be picked up after several rounds of cell division. In another research effort, it was discovered that QD-RGD peptide conjugates can label human mesenchymal stem cells as they differentiate into adipocytes, chondrocytes, and osteoblasts without significantly altering how cells operate generally. QD has proven to be able to bind synuclein and amyloid protein. It can help with the creation of QD-based methods for identifying amyloid clumps inside cells, a critical step in the recognition of neurodegenerative disorders.
Bio-imaging Uses in QD for Cancer Detection
It is well established that a timely cancer diagnosis is essential for effective therapy. New sophisticated solutions continue to be the major area of study in this discipline because of the flaws in current clinical diagnosis techniques. Semiconductor nanocrystals with large surface areas, such as and, are ideal for attaching chemical groups needed for antibody interaction. They are thought to be appropriate for the construction of nanotechnology for the early detection and treatment of disease. When employed as colorants for optical tomography or contrast substances for magnetic resonance imaging, silicon NPs show high efficacy.(39) The procedure of blood flow, also known as angiogenesis, is crucial for the formation and development of tumors. Basic VEGF and FGF, signaling enzymes generated by tumor cells, are what power its processes. Therefore, the initial step in the early identification of a tumor might be the recognition of FGF and VEGF utilizing fluorescent tags. Additionally, it has been observed that the and are interfacial adhesion proteins that fluorescence nanocrystals with antibody conjugates target. Two prostatic tumor cell characteristics,and , were examined in this work. NP-antibody conjugates were used to stain cells, enabling luminescence microscopy to identify the spread of tags and evaluate their numerical ratio.
The growth of QD-based techniques for early prostate tumor detection is anticipated to utilize androgen receptors Ki-67, insulin-like growth factor 1 antibody, p27, CD10, and MTA1 receptor. The epithelium antigen is a helpful tumor diagnostic in the identification and treatment of ovarian tumor. It demonstrates the use of QD with a 610 nm wavelength to accurately and specifically detect CA 125 in cancerous ovarian specimens.(40) It explores human HER 2 in breast tumor identification, with overexpression in 30-35 % of cases. Transferrin and anti-claudin-4 were used for imaging pancreatic tumor cells employing for enhanced light emission.
To get over these restrictions, researchers are working to create FPs with maximal luminescence wavelengths in the NIR region and the ability to concentrate specifically in diseased tissues. The most promising materials for tumor imaging are semiconductor nanocrystals with peak emission wavelengths in the 750–850 nm range and high fluorescent quantum efficiency.(41) Because electromagnetic radiation with a wavelength between 670 and 970 nm can penetrate deeply into biological tissue, it can be effectively used for intravitaltumor imaging with fluorescent tomography methods.
Ag-Based QDs for Bioimaging Techniques
Semiconductor NPs have distinct fluorescence characteristics from commonly used organic fluorophores, making them an attractive tool for bioimaging. It enables the development of efficient biomarkers, biosensors, and anticancer drug delivery systems for specific cells. A broad absorption spectrum, strong photo stability, and high QY of luminescence are among QDs’ optoelectronic characteristics. Compared to organic dyes, quantum dots have several benefits. They are best suited for multiplex imaging due to their narrow size distribution and single-light emission.(42)
CONCLUSIONS
QD has a lot of advantages over organic fluorophores due to their particular optical and chemical properties, making them suited for use as glowing labels in a variety of biological applications. The processes of biological QD production are now being investigated in great detail but are mostly unknown. Based on the particles are located, biological synthesis is split into two processes. Considering the addition of silicon nanocrystals may increase the specificity of many biological parameters, developing hypersensitive techniques for rapid identification of proteins, cells, and genes is one of the main difficulties facing current nanobiotechnology. Nanocrystals offer a promising foundation for nondestructive imaging of proteins, nucleic acids, and tracking molecules, enabling real-time detection and monitoring of their relationship and growth.
For reliable evaluation and the creation of novel, potent medications, it is essential to have the capacity to measure a wide range of biological characteristics with precision at the resolution of discrete molecules. QD’s near-term potential is linked to new particle generations with modest “monodisperse dimensions” and diverse optical characteristics. It is crucial for long-wave infrared spectral NPs since their enormous size has made internal labeling with them difficult.
REFERENCES
1. Omran BA, Whitehead KA, Baek KH. One-pot bioinspired synthesis of fluorescent metal chalcogenide and carbon quantum dots: Applications and potential biotoxicity. Colloids and Surfaces B: Biointerfaces. 2021 Apr 1;200:111578. https://doi.org/10.1016/j.colsurfb.2021.111578
2. Tusher MM. Microbial synthesis of Cadmium selenide quantum dots (CdSe QDs), influencing factors and applications. Optical and Quantum Electronics. 2023 Apr;55(4):332. https://doi.org/10.1007/s11082-023-04632-z
3. Adegoke O, Montaseri H, Nsibande SA, Forbes PB. Organometallic synthesis, structural and optical properties of CdSe quantum dots passivated with ternary AgZnS alloyed shell. Journal of Luminescence. 2021 Jul 1;235:118049. https://doi.org/10.1016/j.jlumin.2021.118049
4. Falsafi SR, Rostamabadi H, Assadpour E, Jafari SM. Morphology and microstructural analysis of bioactive-loaded micro/nanocarriers via microscopy techniques; CLSM/SEM/TEM/AFM. Advances in Colloid and Interface Science. 2020 Jun 1;280:102166. https://doi.org/10.1016/j.cis.2020.102166
5. Jeevanandam J, Kiew SF, Boakye-Ansah S, Lau SY, Barhoum A, Danquah MK, Rodrigues J. Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale. 2022;14(7):2534-71. https://doi.org/10.1039/D1NR08144F
6. Grasso G, Zane D, Dragone R. Microbial nanotechnology: challenges and prospects for green biocatalytic synthesis of nanoscale materials for sensoristic and biomedical applications. Nanomaterials. 2019 Dec 18;10(1):11. https://doi.org/10.3390/nano10010011
7. Liu J, Zheng D, Zhong L, Gong A, Wu S, Xie Z. Biosynthesis of biocompatibility Ag2Se quantum dots in Saccharomyces cerevisiae and its application. Biochemical and Biophysical Research Communications. 2021 Mar 12;544:60-4. https://doi.org/10.1016/j.bbrc.2021.01.071
8. Borovaya M, Horiunova I, Plokhovska S, Pushkarova N, Blume Y, Yemets A. Synthesis, properties and bioimaging applications of silver-based quantum dots. International Journal of Molecular Sciences. 2021 Nov 11;22(22):12202. https://doi.org/10.3390/ijms222212202
9. Shi K, Xu X, Li H, Xie H, Chen X, Zhan Y. Biosynthesized quantum dots as improved biocompatible tools for biomedical applications. Current Medicinal Chemistry. 2021 Jan 1;28(3):496-513. https://doi.org/10.2174/0929867327666200102122737
10. Zhang Y, Xiao JY, Zhu Y, Tian LJ, Wang WK, Zhu TT, Li WW, Yu HQ. Fluorescence sensor based on biosynthetic CdSe/CdS quantum dots and liposome carrier signal amplification for mercury detection. Analytical chemistry. 2020 Feb 5;92(5):3990-7.
11. Alavi M. Biosynthesis of Quantum Dots and Their Therapeutic Applications in the Diagnosis and Treatment of Cancer and SARS-CoV-2. https://doi.org/10.34172/apb.2023.065
12. Xu Y, Musumeci V, Aymonier C. Chemistry in supercritical fluids for the synthesis of metal nanomaterials. Reaction Chemistry & Engineering. 2019;4(12):2030-54. https://doi.org/10.1039/C9RE00290A
13. Yang W, Li X, Fei L, Liu W, Liu X, Xu H, Liu Y. A review on sustainable synthetic approaches toward photoluminescent quantum dots. Green Chemistry. 2022;24(2):675-700.
14.Dok, A.R., Legat, T., de Coene, Y., Van der Veen, M.A., Verbiest, T. and Van Cleuvenbergen, S., 2021. Nonlinear optical probes of nucleation and crystal growth: recent progress and future prospects. Journal of Materials Chemistry C, 9(35), pp.11553-11568. https://doi.org/10.1039/D1GC02964A
15. Menazea AA, Abdelghany AM, Osman WH, Hakeem NA, Abd El-Kader FH. Precipitation of silver nanoparticles in silicate glasses via Nd: YAG nanosecond laser and its characterization. Journal of Non-Crystalline Solids. 2019 Jun 1;513:49-54. https://doi.org/10.1016/j.jnoncrysol.2019.03.018
16. Li W, Kaminski Schierle GS, Lei B, Liu Y, Kaminski CF. Fluorescent nanoparticles for super-resolution imaging. Chemical Reviews. 2022 Jun 27;122(15):12495-543.
17. Budagosky JA, García-Cristóbal A. Multiscale Kinetic Monte Carlo Simulation of Self-Organized Growth of GaN/AlN Quantum Dots. Nanomaterials. 2022 Sep 2;12(17):3052. https://doi.org/10.3390/nano12173052
18. Lekha DC, Shanmugam R, Madhuri K, Dwarampudi LP, Bhaskaran M, Kongara D, Tesfaye JL, Nagaprasad N, Bhargavi VN, Krishnaraj R. Review on silver nanoparticle synthesis method, antibacterial activity, drug delivery vehicles, and toxicity pathways: recent advances and future aspects. Journal of Nanomaterials. 2021;2021(1):4401829. https://doi.org/10.1155/2021/4401829
19. Wang M, Zhang Y, Yao Q, Ng M, Lin M, Li X, Bhakoo KK, Chang AY, Rosei F, Vetrone F. Morphology control of lanthanide doped NaGdF4 nanocrystals via one-step thermolysis. Chemistry of Materials. 2019 Jun 3;31(14):5160-71.
20. Liang T, Qiu X, Ye X, Liu Y, Li Z, Tian B, Yan D. Biosynthesis of selenium nanoparticles and their effect on changes in urinary nanocrystallites in calcium oxalate stone formation. 3 Biotech. 2020 Jan;10:1-6. https://doi.org/10.1007/s13205-019-1999-7
21. Theiss S, Voggel M, Schlötter M, Sutter S, Stöckl MT, Polarz S. Tolerance in superstructures formed from high-quality colloidal ZnO nanoparticles with hexagonal cross-section. CrystEngComm. 2019;21(34):5137-44. https://doi.org/10.1039/C9CE00811J
22. Park SY, Lee S, Yang J, Kang MS. Patterning quantum dots via photolithography: a review. Advanced Materials. 2023 Oct;35(41):2300546. https://doi.org/10.1002/adma.202300546
23. Efros AL, Brus LE. Nanocrystal quantum dots: from discovery to modern development. ACS nano. 2021 Apr 8;15(4):6192-210.
24. Shalabayev Z, Baláž M, Khan N, Nurlan Y, Augustyniak A, Daneu N, Tatykayev B, Dutková E, Burashev G, Casas-Luna M, Džunda R. Sustainable synthesis of cadmium sulfide, with applicability in photocatalysis, hydrogen production, and as an antibacterial agent, using two mechanochemical protocols. Nanomaterials. 2022 Apr 7;12(8):1250. https://doi.org/10.3390/nano12081250
25. Palimi MJ, Tang Y, Alvarez V, Kuru E, Li DY. Green corrosion inhibitors for drilling operation: New derivatives of fatty acid-based inhibitors in drilling fluids for 1018 carbon steel in CO2-saturated KCl environments. Materials Chemistry and Physics. 2022 Sep 1;288:126406. https://doi.org/10.1016/j.matchemphys.2022.126406
26. Alsaggaf, M.S., Elbaz, A.F., El-baday, S. and Moussa, S.H., 2020. Anticancer and antibacterial activity of cadmium sulfide nanoparticles by Aspergillus niger. Advances in Polymer Technology, 2020, pp.1-13.
27. Plastun IL, Zakharov AA, Naumov AA. Features of silver sulfide nanoparticles bacterial synthesis: Molecular modeling. In2020 International Conference on Actual Problems of Electron Devices Engineering (APEDE) 2020 Sep 24 (pp. 318-322). IEEE. doi: 10.1109/APEDE48864.2020.9255570.
28. Amor-Gutiérrez O, Iglesias-Mayor A, Llano-Suárez P, Costa-Fernández JM, Soldado A, Podadera A, Parra F, Costa-García A, de la Escosura-Muñiz A. Electrochemical quantification of Ag 2 S quantum dots: Evaluation of different surface coating ligands for bacteria determination. Microchimica Acta. 2020 Mar;187:1-1. https://doi.org/10.1007/s00604-020-4140-z
29. Munyai S, Tetana ZN, Mathipa MM, Ntsendwana B, Hintsho-Mbita NC. Green synthesis of Cadmium Sulphide nanoparticles for the photodegradation of Malachite green dye, Sulfisoxazole and removal of bacteria. Optik. 2021 Dec 1;247:167851. https://doi.org/10.1016/j.ijleo.2021.167851
30. Yoon J, Oh SG. Synthesis of amine modified ZnO nanoparticles and their photocatalytic activities in micellar solutions under UV irradiation. Journal of Industrial and Engineering Chemistry. 2021 Apr 25;96:390-6. https://doi.org/10.1016/j.jiec.2021.01.043
31. Lin Q, Liang S, Wang J, Zhang R, Wang X. Cadmium sulfide 3D photonic crystal with hierarchically ordered macropores for highly efficient photocatalytic hydrogen generation. Inorganic Chemistry. 2022 Feb 1;61(6):2920-8.
32. Du T, Cai K, Han H, Fang L, Liang J, Xiao S. Probing the interactions of CdTe quantum dots with pseudorabies virus. Scientific Reports. 2015 Nov 10;5(1):16403.
33. Hu Z, Song B, Xu L, Zhong Y, Peng F, Ji X, Zhu F, Yang C, Zhou J, Su Y, Chen S. Aqueous synthesized quantum dots interfere with the NF-κB pathway and confer anti-tumor, anti-viral and anti-inflammatory effects. Biomaterials. 2016 Nov 1;108:187-96. https://doi.org/10.1016/j.biomaterials.2016.08.047
34. Nie X, Jiang C, Wu S, Chen W, Lv P, Wang Q, Liu J, Narh C, Cao X, Ghiladi RA, Wei Q. Carbon quantum dots: A bright future as photosensitizers for in vitro antibacterial photodynamic inactivation. Journal of Photochemistry and Photobiology B: Biology. 2020 May 1;206:111864. https://doi.org/10.1016/j.jphotobiol.2020.111864
35. Kumari A, Sharma A, Sharma R, Malairaman U, Singh RR. Biocompatible and fluorescent water based NIR emitting CdTe quantum dot probes for biomedical applications. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2021 Mar 5;248:119206. https://doi.org/10.1016/j.saa.2020.119206
36. Dassanayake RS, Wansapura PT, Tran P, Hamood A, Abidi N. Cotton cellulose-CdTe quantum dots composite films with inhibition of biofilm-forming S. aureus. Fibers. 2019 Jun 19;7(6):57. https://doi.org/10.3390/fib7060057
37. Priyadarshini E, Priyadarshini SS, Cousins BG, Pradhan N. Metal-Fungus interaction: Review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosphere. 2021 Jul 1;274:129976. https://doi.org/10.1016/j.chemosphere.2021.129976
38. Chugh M, Kumar L, Bhardwaj D, Bharadvaja N. Bioaccumulation and detoxification of heavy metals: an insight into the mechanism. InDevelopment in wastewater treatment research and processes 2022 Jan 1 (pp. 243-264). Elsevier. https://doi.org/10.1016/B978-0-323-85657-7.00013-4
39. Liu Y, He G, He T, Saleem M. Signaling and detoxification strategies in plant-microbes symbiosis under heavy metal stress: a mechanistic understanding. Microorganisms. 2022 Dec 26;11(1):69. https://doi.org/10.3390/microorganisms11010069
40. Priyadarshini E, Priyadarshini SS, Pradhan N. Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Applied Microbiology and Biotechnology. 2019 Apr 2;103:3297-316. https://doi.org/10.1007/s00253-019-09685-3
41. Tarfeen N, Nisa KU, Hamid B, Bashir Z, Yatoo AM, Dar MA, Mohiddin FA, Amin Z, Ahmad RA, Sayyed RZ. Microbial remediation: a promising tool for reclamation of contaminated sites with special emphasis on heavy metal and pesticide pollution: a review. Processes. 2022 Jul 12;10(7):1358. https://doi.org/10.3390/pr10071358
42. Ding C, Huang Y, Shen Z, Chen X. Synthesis and bioapplications of Ag2S quantum dots with near‐infrared fluorescence. Advanced Materials. 2021 Aug;33(32):2007768. https://doi.org/10.1002/adma.202007768
FINANCING
The authors did not receive financing for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Prerak Sudan, Tanveer Ahmad Wani, Shashikant Patil, Ambika Sahoo, Kollathur Sudheer, B Reddy.
Drafting - original draft: Prerak Sudan, Tanveer Ahmad Wani, Shashikant Patil, Ambika Sahoo, Kollathur Sudheer, B Reddy.
Writing - proofreading and editing: Prerak Sudan, Tanveer Ahmad Wani, Shashikant Patil, Ambika Sahoo, Kollathur Sudheer, B Reddy.