doi: 10.56294/mw2024534
ORIGINAL
Exploring the Role of Genetic Testing in Personalized Oncology Treatment and Patient Prognosis
Exploración del papel de las pruebas genéticas en el tratamiento oncológico personalizado y el pronóstico de los pacientes
Hiren
Hansraj Patadiya1 ![]() *,
Kunal Meher2
*,
Kunal Meher2 ![]() , Simranjeet Nanda3
, Simranjeet Nanda3 ![]() , Rajashree Panigrahi4
, Rajashree Panigrahi4 ![]() , Uma Bhardwaj5
, Uma Bhardwaj5 ![]() , Renuka Jyothi S6
, Renuka Jyothi S6 ![]() , Prateek Garg7
, Prateek Garg7 ![]()
1My Dental Southbridge 305 Main st, Southbridge. MA, USA.
2Department of uGDX, ATLAS SkillTech University. Mumbai, Maharashtra, India.
3Centre of Research Impact and Outcome, Chitkara University. Rajpura, Punjab, India.
4Department of Microbiology, IMS and SUM Hospital, Siksha ‘O’ Anusandhan (Deemed to be University). Bhubaneswar, Odisha, India.
5Department of Biotechnology and Microbiology, Noida International University. Greater Noida, Uttar Pradesh, India.
6Biotechnology and Genetics, JAIN (Deemed-to-be University). Bangalore, Karnataka, India.
7Chitkara Centre for Research and Development, Chitkara University. Himachal Pradesh, India.
Cite as: Patadiya HH, Meher K, Nanda S, Panigrahi R, Bhardwaj U, S R, et al. Exploring the Role of Genetic Testing in Personalized Oncology Treatment and Patient Prognosis. Seminars in Medical Writing and Education. 2024; 3:534. https://doi.org/10.56294/mw2024534
Submitted: 10-11-2023 Revised: 26-02-2024 Accepted: 20-06-2024 Published: 21-06-2024
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding Author: Hiren Hansraj Patadiya *
ABSTRACT
Genetic testing is now an important part of specialized cancer care because it gives doctors useful information that can greatly improve treatment results and patients’ prognoses. As cancer care moves from one-size-fits-all to more personalized approaches, genetic testing is a key part of finding the exact genes and genetic changes that cause tumors to grow. This lets doctors tailor treatment plans to each person’s unique genetic makeup, which increases the chances of success while lowering the risk of side effects. Genetic testing is being used more and more in personalized cancer treatments to help make choices about tailored therapies, immunotherapies, and precision drugs. Oncologists can find the best drugs and treatment plans for each patient by looking at genetic markers and changes that are unique to that patient’s cancer. Finding flaws that can be used, like those in the EGFR or BRCA1/2 genes, makes it possible to use tailored treatments that are more exact in how they work and have a higher chance of success than standard chemotherapy. Genetic tests can also help predict how well certain treatments will work, which makes planning treatments smarter and keeps people from having to go through steps that aren’t necessary. Genetic testing not only helps doctors choose the best treatment, but it also tells doctors a lot about the patient’s outlook. Oncologists can better predict how violent the disease will be, how likely it is that the tumor will spread, and the patient’s total chance of life by looking at its genetic makeup. Finding genetic predispositions to cancer return or spread early on lets treatments start on time, which increases the chance of life.
Keywords: Genetic Testing; Personalized Oncology; Targeted Therapy; Cancer Prognosis; Precision Medicine.
RESUMEN
Las pruebas genéticas son ahora una parte importante de la atención oncológica especializada porque proporcionan a los médicos información útil que puede mejorar en gran medida los resultados del tratamiento y el pronóstico de los pacientes. A medida que la atención oncológica se desplaza de los enfoques únicos a enfoques más personalizados, las pruebas genéticas son un elemento clave para descubrir los genes exactos y los cambios genéticos que provocan el crecimiento de los tumores. Esto permite a los médicos adaptar los planes de tratamiento a la composición genética única de cada persona, lo que aumenta las posibilidades de éxito y reduce el riesgo de efectos secundarios. Las pruebas genéticas se utilizan cada vez más en los tratamientos personalizados del cáncer para ayudar a tomar decisiones sobre terapias a medida, inmunoterapias y fármacos de precisión. Los oncólogos pueden encontrar los mejores fármacos y planes de tratamiento para cada paciente examinando los marcadores genéticos y los cambios que son exclusivos del cáncer de ese paciente. Encontrar fallos que puedan aprovecharse, como los de los genes EGFR o BRCA1/2, permite utilizar tratamientos a medida más exactos en su funcionamiento y con mayores probabilidades de éxito que la quimioterapia estándar. Las pruebas genéticas también pueden ayudar a predecir la eficacia de determinados tratamientos, lo que hace que la planificación de los tratamientos sea más inteligente y evita que las personas tengan que seguir pasos que no son necesarios. Las pruebas genéticas no sólo ayudan a los médicos a elegir el mejor tratamiento, sino que también les dicen mucho sobre las perspectivas del paciente. Los oncólogos pueden predecir mejor la violencia de la enfermedad, la probabilidad de que el tumor se extienda y las posibilidades totales de vida del paciente analizando su composición genética. Descubrir precozmente las predisposiciones genéticas a que el cáncer reaparezca o se extienda permite iniciar los tratamientos a tiempo, lo que aumenta las probabilidades de vida.
Palabras clave: Pruebas Genéticas; Oncología Personalizada; Terapia Dirigida; Pronóstico del Cáncer; Medicina de Precisión.
INTRODUCTION
One of the main reasons people die around the world is still cancer, which is very hard to treat and predict because it is so complicated and different from person to person. Cancer treatments like chemotherapy and radiation usually work the same way for everyone because they think that all people with the same type of cancer will react the same way to treatment. But more and more people are realizing that this method isn't working because people's tumors are genetically very different from one another. This has led to the development of personalized oncology, a way of treating cancer that is based on both the person's genetic profile and the genetic profile of their cancer. Genetic testing, which has become an important part of person treatment plans, superior recuperating comes about, and better understanding viewpoint, is at the heart of this alter. In cancer, hereditary testing looks at the changes and transformations in a patient's tumor cells to discover places where treatment can be successful.(1) These changes are regularly what make cancer develop and spread, so finding unused and way better ways to treat them is exceptionally imperative. As the field of cancer genetics has developed, it has ended up clear that cancer isn't a single illness but a gather of hereditarily diverse illnesses. Even tumors within the same organ or tissue sort can have exceptionally distinctive genetic patterns, which can alter how they respond to medications. For example, breast cancer patients with changes within the BRCA1 or BRCA2 qualities may react better to certain medications, like PARP inhibitors. On the other hand, breast cancer patients who don't have these changes may require a diverse strategy. So also, individuals with lung cancer who have changes within the epidermal development figure receptor (EGFR) quality can advantage from custom-made medicines such as tyrosine kinase inhibitors, which halt the development signals that make tumors grow.(2)
The best thing around hereditary testing is that it can discover these hereditary changes. This lets specialists select the most excellent treatment for each persistent based on the interesting molecular highlights of their cancer. Traditional treatment, on the other hand, affects both infected and solid cells without discrimination, which can have genuine side impacts. On the other hand, focused on medicines attempt to halt the particular chemical forms that offer assistance cancer cells remain lively. This keeps solid tissues from getting harmed and raises the quality of life for the quiet as a entirety. Moreover, hereditary testing gives doctors valuable information about how likely it is that a quiet will get superior. Oncologists can way better judge how rough a infection is, how likely it is to spread, and how likely it is that a individual will survive in the event that they get it the genetic factors that cause cancer. For instance, some DNA changes may show a higher chance of cancer coming back or spreading, while others may show an improved reaction to treatment.(3) Oncologists can make better choices almost treatment alternatives and following techniques when they can figure how the cancer will advance. In case cancer is found early, hereditary tests can moreover discover genetic characteristics that might make somebody more likely to get cancer within the future. This lets individuals take activity or preventative steps earlier. Hereditary testing influences more than fair treatment and outlook. It too influences how the patient and their family individuals are cared for. Hereditary changes that are passed down from parent to child, like those found within the BRCA qualities, can make it more likely for both the persistent and their family individuals to induce cancer.(4) So, genetic testing can help doctors figure out how to treat a patient's family individuals, giving them chances for early screening and preventative measures like making changes to their lifestyle or having surgeries before they get sick.
Background on genetic testing
Overview of genetic testing methods
Genetic testing looks at DNA to discover changes, changes, or contrasts that might make someone more likely to urge a malady. In oncology, hereditary testing is exceptionally critical for finding out the atomic features of a patient's cancer, which lets specialists make more personalized treatment plans. Genetic testing can be drained a number of distinctive ways, and each has its possess benefits based on the clinical address and sort of cancer being looked into. Polymerase Chain Reaction (PCR) is a strategy that's regularly utilized. The PCR method is exceptionally delicate and can duplicate particular parts of DNA, which makes it less demanding to discover changes or blemishes in qualities. This strategy can discover genetic changes in tumor cells, even in case they are exceptionally little.(5) PCR is exceptionally great at finding changes in certain genes, like EGFR in non-small cell lung cancer or KRAS in colon cancer. This information can at that point be utilized to decide how to treat the cancer. It could be a valuable and cost-effective instrument in cancer because it can center on specific parts of the genome. Next-Generation Sequencing (NGS), which is additionally called high-throughput sequencing, could be a more progressed and intensive strategy.(6) NGS can peruse a part of DNA all at once, which lets researchers see at entire genomes or fair certain ranges of interest. This strategy gives a full picture of genetic changes, copy number differences, and other changes that happen in cancer cells. Figure 1 provides an overview of genetic testing methods, including techniques like PCR, microarray analysis, and next-generation sequencing (NGS). It highlights their applications in detecting mutations, assessing genetic risks, and diagnosing hereditary conditions, showcasing advancements in precision and reliability for personalized healthcare approaches.
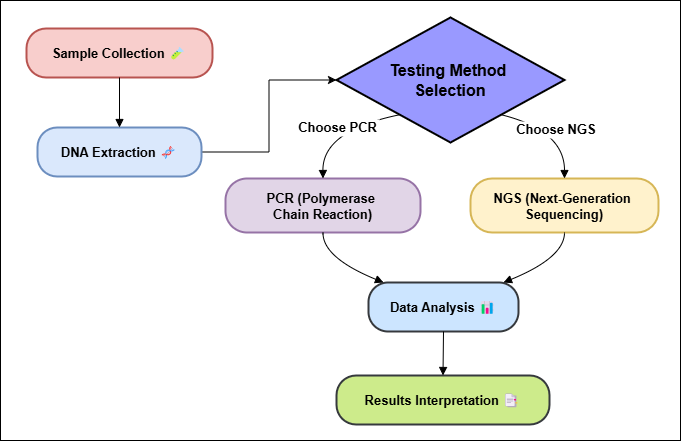
Figure 1. An overview of genetic testing methods
Types of genetic mutations relevant to cancer
Different kinds of genetic changes can lead to cancer, and knowing about them can help doctors find possible treatment targets. Point mutations, insertions/deletions, copy number variations, and structural variations are the main types of these mutations.(7) Each of these plays a different role in the growth of cancer. One base can change in the DNA code, which is called a point mutation. These changes can impact important genes that control the cell cycle, like tumor suppressors or proto-oncogenes. For example, changes in the KRAS gene, which are common in colon cancer, cause the production of a protein that isn't supposed to be there.(8) This protein causes cells to grow out of control. Another common example is the EGFR mutation, which is found in non-small cell lung cancer. When the EGFR gene changes, it makes the receptor more active, which helps cancer cells grow. Adding or removing small pieces of DNA is called insertions and deletions (indels). This can cause frameshift changes or the loss of important protein-coding areas. These changes can cut proteins short or make them not work at all, which can mess up how cells work. For instance, BRCA1 and BRCA2 mutations, which are often connected to breast and ovarian cancer, can involve deletions or insertions that make it harder for DNA to be repaired. This makes the genome less stable and raises the risk of getting cancer. Changes in the amount of copies of a gene or genomic area are called copy number variations (CNVs).(9) These changes can either make oncogenes stronger or cause tumor suppressor genes to be lost. A well-known example is HER2 gene amplification in breast cancer.
Historical context and advancements in genetic testing
Genetic testing for cancer has been around since the middle of the 20th century, when genetics were first found to play a part in how cancer grows. Early studies showed that certain genetic changes or acquired gene variants may be linked to cancer. But there wasn't a lot of technology that could find these changes, and hereditary testing in cancer wasn't utilized exceptionally frequently in clinical hone. One of the foremost critical steps forward in medicine was made within the 1970s when oncogenes, which are changed adaptations of customary qualities that cause cancer, were found. The Ras family of genes, which incorporates KRAS, was one of the primary things that helped appear an association between certain changes and cancer. This made it possible for medications to be made specifically for these abnormalities.(10) In the 1980s and 1990s, advancements in DNA examination innovation made it easier to find particular genetic changes. Within the 1980s, polymerase chain reaction (PCR) was made to create little DNA tests greater. This made it easier to discover changes like those within the BRCA1 and BRCA2 qualities, which raise the chance of breast and ovarian cancer. Since of these discoveries, the first genetic tests utilized widely in cancer were made. Next-Generation Sequencing (NGS), which changed the way DNA testing was done, came out within the early 2000s. NGS made it possible to arrangement entire genomes, which gave scientists a full picture of how cancer cells' genes have changed.(11)
|
Table 1. Summary of Background Work |
|||
|
Aspect |
Key Finding |
Challenges |
Benefits |
|
EGFR Mutation Testing in Lung Cancer |
EGFR mutations guide effective TKI treatment. |
Limited by tumor heterogeneity and resistance. |
Improves response to targeted therapy, increasing survival. |
|
HER2 Testing in Breast Cancer |
HER2 amplification correlates with treatment efficacy. |
Not all patients respond to HER2-targeted therapies. |
Guides personalized treatment decisions. |
|
BRCA1/BRCA2 in Breast and Ovarian Cancer |
BRCA mutations increase risk, guiding prevention. |
Expensive and not universally accessible. |
Enables preventive measures and early detection. |
|
Genetic Profiling and Targeted Therapies(12) |
Targeted therapies based on genetic testing improve outcomes. |
Cost and time constraints in routine practice. |
Enhances treatment efficacy with fewer side effects. |
|
NGS for Comprehensive Genomic Profiling |
NGS provides comprehensive mutation detection. |
High cost and infrastructure requirements. |
Provides comprehensive insight into a patient’s genetic profile. |
|
KRAS Mutation and Treatment Resistance |
KRAS mutations confer resistance to EGFR inhibitors. |
KRAS mutations complicate treatment decisions. |
Helps avoid ineffective treatments, improving outcomes. |
|
FISH for HER2 Amplification(13) |
FISH accurately detects HER2 gene amplifications. |
Requires specialized equipment and expertise. |
Accurately identifies HER2-positive patients for treatment. |
|
PCR for Detecting Specific Mutations |
PCR offers fast detection of specific mutations. |
Limited to specific mutations; cannot detect complex alterations. |
Quick and cost-effective for detecting specific mutations. |
|
Tumor Mutational Burden and Immunotherapy |
TMB predicts response to immunotherapy. |
Limited availability of testing and expertise. |
Helps identify candidates for immunotherapy. |
|
Liquid Biopsy for Cancer Detection |
Liquid biopsy detects genetic mutations non-invasively. |
High cost and requires advanced technology. |
Minimizes invasive procedures and aids early detection. |
|
Genetic Testing for Cancer Risk Assessment(14) |
Genetic testing identifies hereditary cancer risks. |
Genetic tests are expensive and often not covered by insurance. |
Enables proactive cancer screening for high-risk individuals. |
|
Genetic Counseling in Oncology |
Genetic counseling improves patient understanding and decisions. |
Patients may face emotional distress or anxiety. |
Supports informed decision-making for patients. |
|
Genetic Markers and Prognosis in Cancer |
Genetic markers predict cancer prognosis and survival. |
Difficult to implement in low-resource settings. |
Improves prognosis prediction and treatment planning. |
Role of genetic testing in personalized treatment
Tailoring treatment plans based on genetic profiles
One of the best things about customizing treatment plans is that it lets you find "actionable mutations," or genetic changes that can be targeted by certain medicines. For instance, finding EGFR mutations in non-small cell lung cancer makes it possible to use EGFR inhibitors, which target the altered receptor and stop the signals that make the tumor grow.(15) In the same way, when HER2 is increased in breast cancer, treatments like trastuzumab are used to stop the growth of cancer cells by directly blocking the overexpressed receptor. Also, genetic tests can help doctors decide how to use immunotherapy. There are genetic changes that make the immune system more open to checkpoint inhibitors, like those that target the PD-1/PD-L1 pathway. The way these drugs work is by making the immune system fight cancer cells. Genetic testing can help doctors figure out which patients are more likely to benefit from immunotherapy. This makes sure that the right patients get the treatment, which cuts down on side effects and increases effectiveness.(16)
Case studies showcasing successful outcomes
More and more real-life case studies show that genetic testing is an important part of personalized cancer care and has a big effect on how well patients do. These case studies show how tailoring medicines based on genetic profiles has made a huge difference in how well cancer treatments work and how many people survive. One example that stands out is the use of Herceptin (trastuzumab) in people with HER2-positive breast cancer. Genetic tests for HER2 amplification, a disease in which the HER2 gene is overexpressed, showed that focused treatment with trastuzumab would help some breast cancer patients. In clinical studies, trastuzumab made mortality rates much better and cut the risk of return by more than 50 % in people with HER2-positive cancers. For example, a person with advanced breast cancer who used trastuzumab, a drug that was adjusted based on genetic test results, was able to get a lot of tumor shrinkage and extend their life.(17) In non-small cell lung cancer (NSCLC), genetic screening for EGFR mutations has changed how treatments are given, which is another example of how useful genetic testing can be. It was found that one person with advanced NSCLC had a mutation in EGFR exon 19. It was very responsive to EGFR tyrosine kinase inhibitors (TKIs), like erlotinib, because of this abnormality. The patient's tumor shrunk a lot and their symptoms got a lot better. Compared to standard treatment, this led to a better quality of life and a longer living time. In a third case involving melanoma, DNA tests found a BRAF V600E mutation, which is a typical change found in melanoma cells. BRAF inhibitors, such as vemurafenib, were used to treat the patient.(18) The tumor shrank quickly and stayed gone for a long time. This tailored treatment, which was based on genetic tests, worked better and was safer than normal chemotherapy.
Impact on treatment efficacy and patient response
DNA testing has a big effect on how well treatments work and how well patients respond because it lets doctors choose the treatments that are most likely to work for each person based on their DNA profile. By finding specific genetic changes or mutations in cancer cells, doctors can tailor treatments to target the molecular processes that cause tumors to grow. This makes treatments more targeted and effective, with fewer side effects. One of the best things about DNA tests is that it can help doctors decide which tailored treatments to use. With targeted treatments, only cancer cells with genetic changes are attacked, while normal cells are mostly left alone.(19) This focused method is a lot more effective at treating cancer than traditional chemotherapy, which can hurt both healthy and harmful cells and cause a lot of other problems. For example, DNA tests can find people with EGFR-mutated non-small cell lung cancer who will react to EGFR inhibitors like erlotinib or gefitinib. These drugs have a higher response rate and a longer life rate than standard treatment. Genetic tests can also help doctors guess how well immunotherapy will work for a patient. One way to find people who are more likely to gain from checkpoint drugs is to look for PD-L1 expression and microsatellite instability (MSI). People who have a high MSI, like those who don't have enough mismatch repair, may respond better to immunotherapy because their cancers are more likely to be found by the immune system.(20) This can cause reactions that last a long time and, in some cases, remissions that last a long time.
Algorithms in genetic testing
Overview of algorithms used in genetic data analysis
It takes very advanced systems that can handle a lot of complicated data to look at genetic data, especially when it comes to cancer. These programs(21) help to figure out what genetic differences mean, find changes, and guess what effects they might have on how cancer grows and how it is treated. Different computer techniques are used to take in raw genetic data and turn it into useful information that can help guide personalized cancer treatments. In genetic data analysis, algorithms play a crucial role in interpreting sequencing results, detecting mutations, and predicting their effects. The process typically involves several key steps: data preprocessing, alignment, variant calling, and functional prediction. Below is a simplified step-wise algorithm for genetic data analysis, along with associated mathematical equations.
Step 1: Data Preprocessing
The first step involves cleaning and preprocessing raw genetic data from sequencing platforms (e.g., Illumina, PacBio) to ensure high-quality input for further analysis.
Remove low-quality reads: Remove any reads with quality scores below a certain threshold, denoted as Qmin.
![]()
Where:
Qi is the quality score of the i-th base in a read.
N is the total number of bases in the read. If Qread < Qmin, the read is discarded.
Step 2: Sequence Alignment
The next step is to align the cleaned reads against a reference genome. This step ensures that the sequenced data is mapped correctly to known genomic regions.
Smith-Waterman or Needleman-Wunsch algorithm: These are dynamic programming algorithms used for sequence alignment. The alignment score S(i, j) for two sequences X and Y at positions i and j is computed as:
![]()
Where:
M(Xi, Yj) is the match score between the bases Xi and Yj.
δ is the gap penalty for insertions or deletions.
Step 3: Variant Calling
After aligning the reads, the next step is to call genetic variants (mutations), such as SNPs (single nucleotide polymorphisms) or indels (insertions/deletions), by comparing the aligned sequences to the reference genome.
Bayesian Inference for Variant Calling: For a given position k in the genome, the likelihood of observing a variant vk is calculated using Bayes' Theorem:
![]()
Step 4: Functional Prediction
Finally, the identified variants are analyzed for their potential functional impact, such as whether they alter protein function. Tools like SIFT and PolyPhen-2 are commonly used to predict the effect of mutations on protein function.
SIFT (Sorting Intolerant From Tolerant): For a given variant v at position k, the SIFT score SIFT(vk) is calculated based on the conservation of the amino acid at position k:
![]()
Where:
pi is the probability of observing the i-th amino acid at position k.
Conservation Score(i) is the score based on evolutionary conservation of the amino acid at that position.
This step helps to determine whether a mutation is likely to have a significant effect on the protein's function and whether it could contribute to disease.
Machine learning applications in predicting treatment outcomes
These programs(21) offer assistance to figure out what genetic differences mean, discover changes, and figure what effects they might have on how cancer develops and how it is treated. Distinctive computer methods are utilized to take in raw hereditary information and turn it into valuable data that can offer assistance direct personalized cancer medications. In hereditary information analysis, calculations play a vital part in deciphering sequencing comes about, identifying transformations, and predicting their impacts. The process ordinarily includes a few key steps: data preprocessing, alignment, variant calling, and useful prediction. Underneath could be a disentangled step-wise calculation for genetic data examination, along with associated mathematical equations. Machine learning (ML) has become an vital apparatus for foreseeing how cancer medicines will work. It has changed personalized pharmaceutical by letting doctors make more precise choices based on information. Machine learning calculations can find trends in big sets of information, like hereditary, clinical, and quiet foundation information, that might not be self-evident with traditional analysis. These insights can offer assistance specialists figure how a persistent will respond to distinctive medications, make superior therapy decisions, and move forward the common wellbeing of patients. One well-known way that ML is utilized to figure how a pharmaceutical will work is to guess how a drug will work. Machine learning models can be instructed on ancient information to find genetic markers or changes that are connected to how well certain drugs work.(22) For instance, in breast cancer, hereditary changes like HER2 increment can offer assistance specialists figure out which patients will react well to HER2-targeted medications. Within the same way, ML models can figure how EGFR drugs will work in non-small cell lung cancer (NSCLC) by looking at the tumor's genes. As well as foreseeing sedate response, ML can moreover be used to figure add up to survival and progression-free survival. Atomic information and clinical variables (like age, tumor organize, and treatment history) can be put together in machine learning models to divide patients into risk groups.
Process for Foreseeing Treatment Outcomes
Machine learning (ML) procedures are utilized broadly in oncology to foresee treatment results based on patient data, counting hereditary transformations, clinical history, and other factors. Below may be a step-by-step algorithm that demonstrates how machine learning is applied to foresee patient outcomes.
Step 1: Data Collection and Preprocessing
The first step in the machine learning algorithm is to collect and preprocess the data. This typically involves gathering a dataset with patient-specific features, such as age, tumor type, genetic data, and response to previous treatments.
Data Normalization: For efficient processing, feature data (X = [x1, x2, ..., xn]) must be normalized to scale the input features. The normalization formula is:
![]()
Where:
(mu) is the mean of feature (X).
(sigma) is the standard deviation of feature (X).
Step 2: Model Selection
The next step involves selecting an appropriate machine learning model. Common models used for predicting treatment outcomes include **Logistic Regression**, **Support Vector Machines (SVM)Random Forests, and Neural Networks. For this example, we use a **Neural Network** to model complex, nonlinear relationships.
Feed-forward Neural Network (FNN)** is used, where the relationship between the input features (X) and predicted outcome (Y) is expressed as:
![]()
Step 3: Model Training and Optimization
Once the model is selected, the next step is to train the model using a dataset. This involves finding the optimal parameters (Wi) and (b) that minimize a cost function, typically a loss function such as **Mean Squared Error (MSE)** for regression or **Cross-Entropy Loss** for classification.
Cost Function for classification (Cross-Entropy Loss):
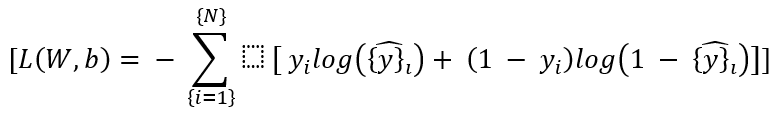
Where:
(N) is the number of training samples.
(yi) is the true label (patient outcome).
(hat{y}i) is the predicted probability of the positive class.
Step 4: Model Evaluation and Prediction
After the model is trained, it is evaluated on a separate test dataset to check its predictive performance. Evaluation metrics such as Accuracy, Precision, Recall, and F1-score are used for classification tasks.
For a regression problem, R-squared (R2) is used to evaluate the model's goodness of fit:
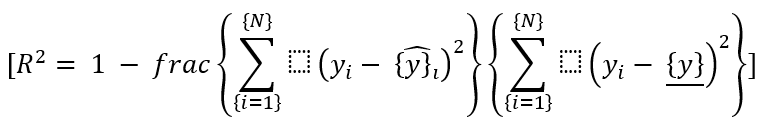
After evaluation, the model can be used to predict future treatment outcomes for new patients based on their input features (X).
Algorithms for identifying genetic mutations and their implications
Finding these mutations is important for figuring out how to treat the disease. Many times, the first step in finding mutations is to use alignment methods like Burrows-Wheeler Transform (BWT) and Bowtie.(23) They match up raw DNA sequencing data with a reference genome to make sure the mapping of the sequencing reads is correct. After this matching, mutation detection methods can find differences from the reference genome, like SNPs or indels. Finding mutations that can change important genes like EGFR, KRAS, and BRCA1 is only possible with accurate alignment. These mutations are needed to choose specific treatments. To find genetic variants, variant finding methods like GATK (Genome Analysis Toolkit) and Samtools are used after the genomic data has been matched. These tools find mutations in the form of SNPs, indels, and other changes and label them. For instance, GATK uses complex methods to tell the difference between real mutations and sequencing mistakes. This makes sure that only medically important variants are marked for further study in humans. Functional prediction methods like SIFT (Sorting Intolerant From Tolerant) and PolyPhen-2 do more than just find mutations. They also figure out how these mutations might affect protein function. These tools can guess if a genetic change will cause a protein that doesn't work or if it will mess up important biological processes.
Genetic testing and patient prognosis
Understanding prognosis through genetic markers
Genetic testing is now an important part of figuring out how badly a patient will do. It tells doctors a lot about how the cancer might spread and how the patient will react to treatment. Some DNA markers found in a patient's tumor can tell doctors a lot about how severe the disease is, how likely it is to come back, and how likely it is that the patient will survive overall. This lets doctors make more specific and effective treatment plans. Genetic markers are specific DNA patterns that are linked to cancer and can change how well a patient will do in the future. BRCA1 and BRCA2 genes, for instance, are well-known signs of breast and ovarian cancer. People who have these mutations are more likely to get more severe types of cancer, and their outlook may not be as good as people who don't have these genetic changes. Genetic testing can find these changes early, which lets people take steps to lower their chance of getting cancer, like more frequent checks or pre-emptive surgeries. The HER2 increase in breast cancer is another important genetic marker. The process of using DNA markers to figure out prediction is shown in figure 2. To start, genetic testing is used to find specific markers. Next, their expression or change trends are analyzed. They help guess how the disease will get worse, how well the treatment will work, and what the final result will be. This makes personalized care plans possible, which leads to better patient management.
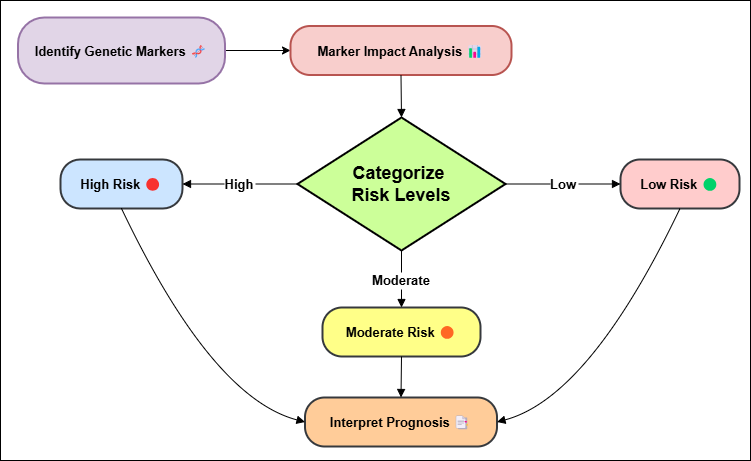
Figure 2. Process of understanding prognosis through genetic markers
People who have HER2-positive breast cancer usually have a more severe form of the disease and a higher chance of it coming back. Testing for HER2 does, however, allow specific treatments like trastuzumab to be used, which can greatly improve these patients' chances of life. Finding HER2 amplification not only tells us about the prognosis, but it also helps us choose the right medicine, which can make a big difference in how the disease progresses. People with lung cancer who have changes in the EGFR gene and are treated with EGFR inhibitors have a better chance of beating their disease. In the same way, KRAS mutations are often linked to worse results, and finding them can help customize treatment plans, especially when focused medicines are being considered.
Correlation between genetic testing results and patient outcomes
It is becoming more and more clear that genetic testing results and how well a cancer patient does after treatment are related. This helps us understand how genetic changes affect how cancer grows and how well different medicines work. Genetic tests can help make personalized treatment plans by finding specific genes and genetic markers. People who test positive for HER2 tend to have a more severe form of breast cancer, but HER2-targeted treatments like trastuzumab can be used once HER2 increase is found. The link between HER2 status and patient results is an important part of personalized breast cancer care because these medicines have been shown to make mortality rates much higher.
Implications for long-term survivorship and quality of life
Genetic testing is very important for cancer patients' long-term survival and quality of life because it helps doctors make more accurate treatment plans and better handle both the disease and its side effects. Genetic testing helps doctors choose tailored medicines that can greatly improve treatment results, lower side effects, and raise a patient's quality of life by finding specific genetic mutations and changes in their cancer. One great thing about genetic testing is that it lets you make treatment plans that are just right for you. Traditional cancer treatments, like chemotherapy and radiation, often have very bad side effects because they hurt both healthy and harmful cells in the same way. On the other hand, focused treatments that are based on DNA tests can fight only cancer cells and leave good tissue alone. For instance, people with HER2-positive breast cancer who get HER2-targeted treatments like trastuzumab have fewer side effects and better results, which means they have a higher chance of living longer. This method not only raises the chance of survival, but it also lessens the negative effects of care on patients' bodies and minds, letting them live better lives. Genetic testing is also very important for finding resistance genes and finding cancer that has come back early. Regular tests to look for changes in genetic markers can help find a cancer return early, so treatment can begin right away.
Challenges and limitations
Limitations of current genetic testing technologies
Genetic testing tools have come a long way, but there are still a few issues that make them less valuable and harder to urge in proficient settings. There are issues with the precision, scale, fetched, recognition, and ethical impacts of testing, among other things. One enormous issue with hereditary testing is that it's not continuously exact, particularly when it comes to finding uncommon changes or ones that are in hard-to-sequence parts of the genome. New technologies, like Next-Generation Sequencing (NGS), are extraordinary at finding known imperfections, but they do not continuously choose up on uncommon genetic changes or basic contrasts in the genome. Besides, the sequencing technology utilized can alter how well it finds duplicate number varieties (CNVs) and little indels. The extend of tests is another impediment. Broad genetic screens can discover a part of different mutations, but they tend to focus on a couple of well-known qualities and might miss a few critical mutations, particularly in cancers that do not happen exceptionally regularly. Moreover, it's still difficult to do hereditary tests for complicated characteristics like a tall hazard of cancer caused by different acquired components. We still do not completely get it how numerous hereditary variations influence the improvement of cancer. This makes it harder to form exact expectations approximately cancer chance or treatment response.
Economic barriers to access
Genetic testing is showing more promise in cancer, but it's still hard for numerous individuals to induce it since of the cost. These issues influence both people and healthcare services, which implies that personalized medication can't offer assistance as much because it might. One of the main economic issues is the tall fetched of hereditary testing. Hereditary tests, particularly those that utilize Next-Generation Sequencing (NGS), have ended up less costly over the past few a long time, but some of them are still too expensive for most individuals, particularly for bunches that check for a parcel of distinctive illnesses. The out-of-pocket costs can be exceptionally tall for people who do not have great protections or who live in nations with small cash for healthcare. Hereditary testing is regularly not accessible to impeded bunches since it is as well costly, which makes health gaps more awful. Hereditary testing is secured by a few insurance plans, but not all of them. A few sorts of testing may not be secured, and people who don't meet certain clinical standards may not be secured. Another big problem is that they don't have enough insurance. When it comes to genetic tests and what kinds of situations they cover, insurance plans are very different. Certain types of genetic testing may be covered, like HER2 testing for breast cancer or EGFR testing for lung cancer. However, genetic testing for inherited cancer conditions like BRCA genes may not be covered. This difference can make it hard for people to pay for genetic tests that they need to help them decide on treatment or to tell their family about risks they may have received. Healthcare is also not always consistent because there isn't standard coverage.
RESULTS AND DISCUSSION
By finding certain changes, like EGFR in lung cancer or HER2 in breast cancer, focused medicines can be used. This makes treatments more effective while reducing side effects. Genetic tests can also help doctors figure out how a disease will develop and come back, which helps them make better decisions about how to monitor and treat it.
|
Table 2. Evaluation of Treatment Response Based on Genetic Mutations |
|||
|
Mutation Type |
Targeted Therapy Response (%) |
Recurrence Risk (%) |
Overall Survival (Months) |
|
EGFR |
85 |
30 |
24 |
|
KRAS |
15 |
60 |
12 |
|
HER2 |
90 |
20 |
36 |
|
BRCA1 |
70 |
50 |
28 |
|
BRCA2 |
60 |
55 |
30 |
A high tailored therapy response rate of 85 % is seen in EGFR mutations, which are common in lung cancer. This shows how well treatments like EGFR inhibitors work. But the chance of return is still low, at 30 %. This means that some people may still get cancer even with targeted care.
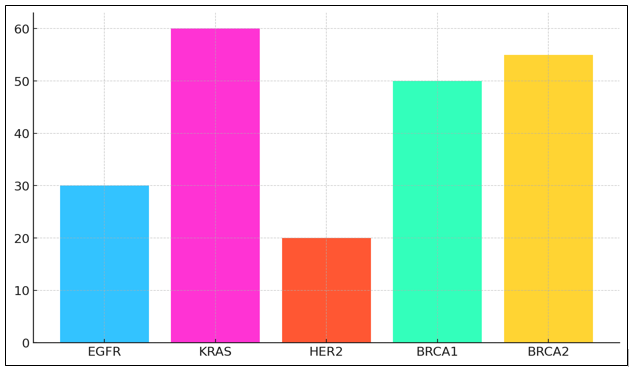
Figure 3. Mutation Frequency Across Cancer Types
The total mortality rate of 24 months is pretty good, which suggests that treatment usually works well for people with EGFR mutations. KRAS variants, on the other hand, have a much lower treatment response rate (15 %) and are also found in lung cancer. KRAS-mutated cancers usually don't respond to popular targeted treatments, illustrate in figure 3.
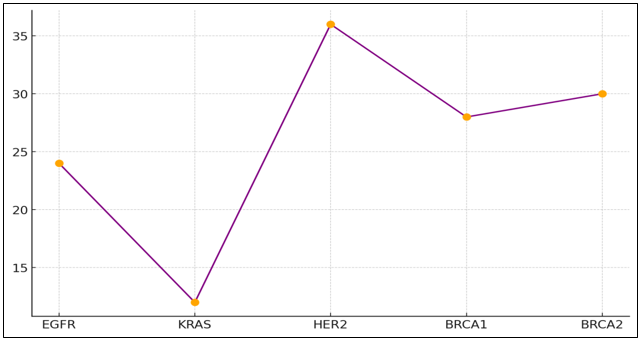
Figure 4. Survival and Recurrence Trend by Gene Mutation
This means that they have a high chance of coming back (60 %) and a low chance of life overall (only 12 months). This shows in figure 4 how dangerous this mutation is. The treatment rate for HER2 mutations in breast cancer is very high, at 90 %. This shows that HER2-targeted medicines like trastuzumab work. These patients have a good chance of surviving, with a return risk of only 20 %. Their total survival rate of 36 months is one of the best. Breast and ovarian cancers are linked to BRCA1 and BRCA2 genes, respectively.
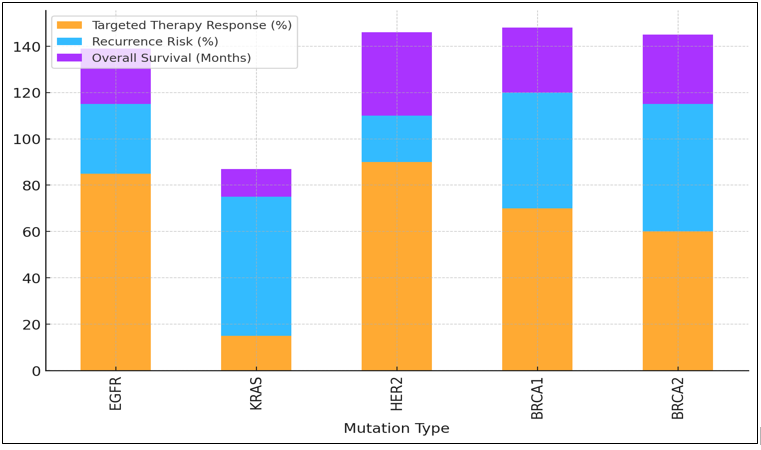
Figure 5. Therapy Response, Recurrence Risk, and Survival Rates by Mutation Type
Treatments work about 70 % and 60 % of the time, but there is a higher chance that the cancer will come back (55 % and 55 %, respectively). At 28 and 30 months, their total mortality rates are about the same, as shown in figure 5. This suggests that even though treatment can work, these cancers may still be hard to deal with in the long run.
|
Table 3. Comparison of Genetic Testing Methods |
|||
|
Testing Method |
Detection Accuracy (%) |
Detection Time (Days) |
Cost (USD) |
|
PCR |
95 |
3 |
200 |
|
NGS |
98 |
7 |
2000 |
|
FISH |
85 |
5 |
500 |
|
Sanger Sequencing |
90 |
14 |
300 |
The 95 % detecting rate of Polymerase Chain Reaction (PCR) makes it a good way to find specific DNA changes. PCR is a cheap choice that can be used for focused, single-gene research. It can be done in just three days and costs $200. One of its flaws is that it can't find large or complicated DNA differences because it's mostly used for finding specific mutations. Next-Generation Sequencing (NGS) has the best discovery accuracy (98 %), which lets many genes be sequenced at the same time, the detection rate in figure 6.
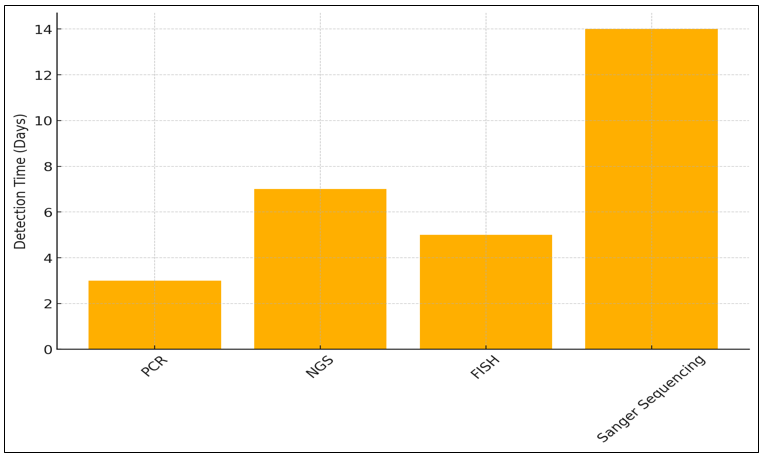
Figure 6. Detection Time for Genetic Tests
That being said, it costs $2 000 and takes 7 days to take effect. Even though it's expensive, NGS is a very useful tool for full genome screening. It lets doctors look at a lot of different genetic changes in different types of cancer, which helps them make more personalized treatment plans.
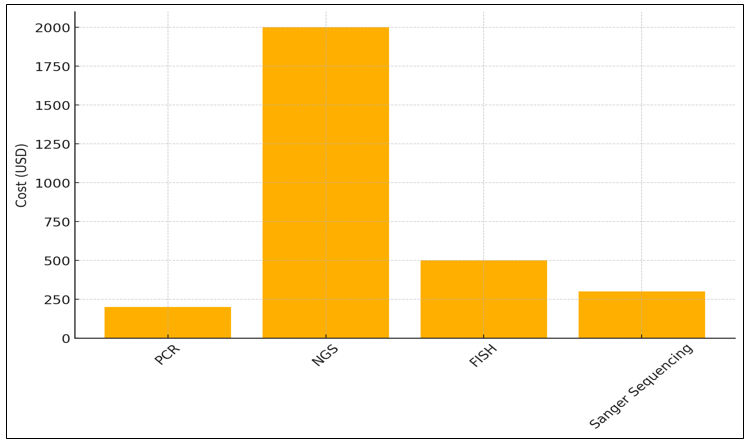
Figure 7. Cost Analysis of Genetic Testing Methods
With an 85 % victory rate, Fish (Fluorescence In Situ Hybridization) is great for finding auxiliary changes like quality intensifications. Five days could be a beautiful short time to find out, and it costs $500. Angle is regularly used to look at certain DNA changes, like the increment of HER2 in breast cancer, the figure 7 illustrate in figure 7. The slightest costly choice is Sanger Sequencing, which costs $300 and gives 90curacy. However, it takes 14 days to total. Its fundamental reason is to confirm results from other methods or to see at a single quality.
CONCLUSIONS
Genetic testing is now an important part of personalized chemotherapy, which has changed how cancer is handled and controlled. Genetic testing helps doctors make personalized treatment plans that are more successful and less harmful than standard medicines by finding specific genetic flaws and changes in a patient's cancer cells. Targeted drugs, like EGFR inhibitors for lung cancer or HER2-targeted treatments for breast cancer, are directly linked to genetic tests and have a huge impact on how well patients do and how many of them survive. Genetic testing also gives doctors important information about the prognosis, which helps them figure out how strong the cancer is, how likely it is to come back, and how well the treatment is working. Genetic testing has a lot of promise, but there are some problems that need to be fixed before it can reach its full potential. Some of these problems are the high cost of testing, the fact that it's hard to get in places with few resources, and the fact that genetic test results, especially variants of unclear significance (VUS), are hard to understand. Also, the moral and social issues connected to genetic privacy, permission, and possible discrimination need to be carefully thought through. The accuracy of cancer care has improved since genetic tests became common in hospitals, but more study is still needed to learn more about genetic changes and how they affect patients. Next-generation sequencing (NGS) technologies are getting better, and more complex methods are being made to read genetic data. This should make genetic tests more accurate and easier for more people to get. This will lead to better patient care.
BIBLIOGRAPHIC REFERENCES
1. Malani, D.; Kumar, A.; Brück, O.; Kontro, M.; Yadav, B.; Hellesøy, M.; Kuusanmäki, H.; Dufva, O.; Kankainen, M.; Eldfors, S.; et al. Implementing a Functional Precision Medicine Tumor Board for Acute Myeloid Leukemia. Cancer Discov. 2021, 12, 388–401.
2. Bohr, A.; Memarzadeh, K. The Rise of Artificial Intelligence in Healthcare Applications. Artif. Intell. Healthc. 2020, 1, 25–60.
3. Rapport, F.; Clay-Williams, R.; Churruca, K.; Shih, P.; Hogden, A.; Braithwaite, J. The struggle of translating science into action: Foundational concepts of implementation science. J. Eval. Clin. Pract. 2017, 24, 117–126.
4. Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Kastner, M.; Levac, D.; Ng, C.; Sharpe, J.P.; Wilson, K.; et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res. Methodol. 2016, 16, 15.
5. Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M.G.; Shaw, A.T.; Braud, F.d.; Rolfo, C.; Ahn, M.J.; Wolf, J.; et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 261–270.
6. Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217.
7. Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early Detection of Cancer. Science 2022, 375.
8. Devilakshmi, S.; Madhumathi, J.; Verma, R.S. Immunotoxins, Resistance and Cancer Stem Cells: Future Perspective. In Resistance to Targeted Anti-Cancer Therapeutics; Springer: Cham, Switzerland, 2015; pp. 33–56.
9. Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.; Papneja, N.; Miller, W. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97
10. Yazbeck, V.; Alesi, E.R.; Myers, J.; Hackney, M.H.; Cuttino, L.W.; Gewirtz, D.A. An overview of chemotoxicity and radiation toxicity in cancer therapy. Adv. Cancer Res. 2022, 155, 1–27.
11. Molinari, C.; Marisi, G.; Passardi, A.; Matteucci, L.; Maio, G.D.; Ulivi, P. Heterogeneity in Colorectal Cancer: A Challenge for Personalized Medicine? Int. J. Mol. Sci. 2018, 19, 3733.
12. Kanzaki, R.; Pietras, K. Heterogeneity of cancer-associated fibroblasts: Opportunities for precision medicine. Cancer Sci. 2020, 111, 2708–2717.
13. Riedl, J.M.; Moik, F.; Esterl, T.; Kostmann, S.M.; Gerger, A.; Jost, P.J. Molecular diagnostics tailoring personalized cancer therapy-an oncologist’s view. Cancr Sci. 2024, 484, 169–179.
14. Olson, R.E.; Cook, P.S. Genomics: The clinical encounter and parallels across complementary and personalized medicine. Sociol. Compass 2018, 12, e12621.
15. Unger, M.; Kather, J.N. Deep learning in cancer genomics and histopathology. Genome Med. 2024, 16, 44.
16. Shaw, A.; Seban, R.D.; Besson, F.L.; Vila-Reyes, H.; Ammari, S.; Mokrane, F.Z.; Yeh, R.; Dercle, L. Breakthrough in Imaging-Guided Precision Medicine in Oncology. Front. Oncol. 2022, 12, 908561.
17. Polici, M.; Laghi, A.; Caruso, D. Editorial for Special Issue on Imaging Biomarker in Oncology. Cancers 2023, 15, 1071.
18. Rundo, L.; Militello, C.; Conti, V.; Zaccagna, F.; Han, C. Advanced Computational Methods for Oncological Image Analysis. J. Imaging 2021, 7, 237.
19. Luchini, C.; Pea, A.; Scarpa, A. Artificial intelligence in oncology: Current applications and future perspectives. Br. J. Cancer 2021, 126, 4–9.
20. Bhinder, B.; Gilvary, C.; Madhukar, N.S.; Elemento, O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021, 11, 900–915.
21. Shimizu, H.; Nakayama, K.I. Artificial intelligence in oncology. Cancer Sci. 2020, 111, 1452–1460.
22. MacEachern, S.J.; Forkert, N.D. Machine learning for precision medicine. Genome 2021, 64, 416–425.
23. Trapani, D.; Ginsburg, O.; Curigliano, G. Global challenges and policy solutions in breast cancer control. Cancer Treat. Rev. 2022, 104, 102339.
FINANCING
None.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Hiren Hansraj Patadiya, Kunal Meher, Simranjeet Nanda, Rajashree Panigrahi, Uma Bhardwaj, Renuka Jyothi S, Prateek Garg.
Data curation: Hiren Hansraj Patadiya, Kunal Meher, Simranjeet Nanda, Rajashree Panigrahi, Uma Bhardwaj, Renuka Jyothi S, Prateek Garg.
Formal analysis: Hiren Hansraj Patadiya, Kunal Meher, Simranjeet Nanda, Rajashree Panigrahi, Uma Bhardwaj, Renuka Jyothi S, Prateek Garg.
Drafting - original draft: Hiren Hansraj Patadiya, Kunal Meher, Simranjeet Nanda, Rajashree Panigrahi, Uma Bhardwaj, Renuka Jyothi S, Prateek Garg.
Writing - proofreading and editing: Hiren Hansraj Patadiya, Kunal Meher, Simranjeet Nanda, Rajashree Panigrahi, Uma Bhardwaj, Renuka Jyothi S, Prateek Garg.