doi: 10.56294/mw2024536
ORIGINAL
Diagnostic and Prognostic Value of Liquid Biopsies in Non-Invasive Cancer Monitoring
Valor diagnóstico y pronóstico de las biopsias líquidas en el seguimiento no invasivo del cáncer
Lokesh Ravilla1 ![]() , Jagmeet Sohal2
, Jagmeet Sohal2 ![]() , Swarna Swetha Kolaventi3
, Swarna Swetha Kolaventi3 ![]() , Divya Sharma4
, Divya Sharma4 ![]() , Niranjan Sahu5
, Niranjan Sahu5 ![]() , Money Saxena6
, Money Saxena6 ![]()
1Centre for Multidisciplinary Research, Anurag University. Hyderabad, Telangana, India.
2Chitkara Centre for Research and Development, Chitkara University. Himachal Pradesh, India.
3Department of uGDX, ATLAS SkillTech University. Mumbai, Maharashtra, India.
4Centre of Research Impact and Outcome, Chitkara University. Rajpura, Punjab, India.
5Department of Radiology, IMS and SUM Hospital, Siksha ‘O’ Anusandhan (Deemed to be University). Bhubaneswar, Odisha, India.
6School of Nursing, Noida International University. Greater Noida, Uttar Pradesh, India.
Cite as: Ravilla L, Sohal J, Kolaventi SS, Sharma D, Sahu N, Saxena M. Diagnostic and Prognostic Value of Liquid Biopsies in Non-Invasive Cancer Monitoring. Seminars in Medical Writing and Education. 2024; 3:536. https://doi.org/10.56294/mw2024536
Submitted: 11-11-2023 Revised: 27-02-2024 Accepted: 20-06-2024 Published: 21-06-2024
Editor: PhD.
Prof. Estela Morales Peralta ![]()
ABSTRACT
Researchers are finding that liquid biopsies, which are samples of blood, saliva, or pee that are not solid, can be used to find biomarkers that can help diagnose and track cancer. Traditional tissue biopsies are invasive and may not always be possible. Liquid biopsies, on the other hand, can be used over and over again and in real time to find genetic changes linked to cancer, differences in tumors, and treatment reactions. This piece talks about the diagnostic and predictive worth of liquid samples in cancer care. It focuses on how they can help with early diagnosis, tracking the development of the disease, figuring out the minimal residual disease (MRD), and guessing how well treatment will work. A liquid biopsy has gotten a lot of attention as an early cancer screening method because it can find genetic changes early on, often before any signs show up. It is possible to find cancer-related changes in cell-free DNA (cfDNA), circulating tumor DNA (ctDNA), and circulating tumor cells (CTCs) using liquid samples. This information can help with early action and specific treatment plans. Liquid biopsies also show differences between tumors and how genes change over time, which is very important for figuring out how drug resistance works. Liquid biopsy has been very helpful for figuring out how well treatment is working and finding relapses. Monitoring ctDNA levels in real time can show how much of a tumor there is, which lets doctors see how well treatment is working and make changes to treatment plans as required. The amount and prevalence of ctDNA have been linked to tumor growth, showing an early sign of recurrence even before regular imaging methods show disease that can be seen by a doctor.
Keywords: Liquid Biopsy; Cancer Detection; Circulating Tumor DNA (Ctdna); Minimal Residual Disease (MRD); Prognostic Monitoring.
RESUMEN
Los investigadores están descubriendo que las biopsias líquidas, que son muestras de sangre, saliva u orina que no son sólidas, pueden utilizarse para encontrar biomarcadores que ayuden a diagnosticar y rastrear el cáncer. Las biopsias de tejido tradicionales son invasivas y no siempre son posibles. Las biopsias líquidas, en cambio, pueden utilizarse una y otra vez y en tiempo real para encontrar cambios genéticos relacionados con el cáncer, diferencias en los tumores y reacciones al tratamiento. Este artículo habla del valor diagnóstico y predictivo de las muestras líquidas en el tratamiento del cáncer. Se centra en cómo pueden ayudar al diagnóstico precoz, seguir el desarrollo de la enfermedad, averiguar la enfermedad mínima residual (EMR) y adivinar lo bien que funcionará el tratamiento. La biopsia líquida ha recibido mucha atención como método de detección precoz del cáncer porque puede detectar cambios genéticos en una fase temprana, a menudo antes de que aparezca ningún signo. Es posible detectar cambios relacionados con el cáncer en el ADN libre de células (cfADN), el ADN tumoral circulante (ctADN) y las células tumorales circulantes (CTC) utilizando muestras líquidas. Esta información puede ayudar a actuar precozmente y a elaborar planes de tratamiento específicos. Las biopsias líquidas también muestran diferencias entre tumores y cómo cambian los genes con el tiempo, lo que es muy importante para averiguar cómo funciona la resistencia a los fármacos. La biopsia líquida ha sido muy útil para determinar la eficacia del tratamiento y detectar recaídas. La monitorización de los niveles de ctADN en tiempo real puede mostrar la cantidad de tumor existente, lo que permite a los médicos comprobar la eficacia del tratamiento y realizar los cambios necesarios en los planes terapéuticos. La cantidad y prevalencia del ctADN se han relacionado con el crecimiento tumoral, mostrando un signo temprano de recurrencia incluso antes de que los métodos de imagen habituales muestren una enfermedad que pueda ser vista por un médico.
Palabras clave: Biopsia Líquida; Detección del Cáncer; ADN Tumoral Circulante (Ctadn); Enfermedad Mínima Residual (EMR); Monitorización Pronóstica.
INTRODUCTION
Cancer is still one of the main reasons of death in the world, and early diagnosis and accurate tracking are key to making things better for patients. Traditional ways of finding cancer, like tissue tests and imaging tools, have helped a lot with finding the disease, figuring out how bad it is, and deciding how to treat it. But these methods often have problems, like being invasive or needing to be done over and over again. They also have trouble finding cancers in their early stages or keeping track of how the disease is progressing in real time. Because of this, liquid biopsy has become a new non-invasive way to diagnose, predict, and keep an eye on cancer, which could help solve many of these problems as well. Liquid biopsy is the method of looking at biomarkers in blood or other body liquids like plasma, pee, or spit. These biomarkers grant hereditary data almost the infection. RNA pieces, circulating tumor DNA (ctDNA), and circulating tumor cells (CTCs) are the most common parts of a fluid biopsy. Among these, ctDNA, which is made up of DNA pieces that tumor cells discharge into the circulation system, has gotten a lot of consideration for its capacity to analyze and foresee cancer. ctDNA can appear the hereditary surrenders, chromosomal changes, and other atomic changes that are happening within the tumor. This lets doctors get a quick look at the tumor's hereditary makeup without having to require invasive tissue tests.(1) As one of the leading things approximately fluid biopsy is that it can discover cancer prior, indeed some time recently any signs appear up. Finding cancer early is exceptionally critical for expanding survival rates, particularly for sorts of cancer where the outlook is closely connected to the arrange at disclosure. Studies have shown that ctDNA research can find mutations and genetic changes linked to different types of cancer, like lung, breast, bowel, and ovarian cancer. This could help doctors find these cancers faster and more accurately.
Tumor heterogeneity(2) or the variety of genes within a tumor and between spread spots, makes planning and keeping track of treatment very difficult. Because traditional tests only show a small part of the tumor, they might not show the full range of genetic changes happening inside the tumor. Liquid biopsy, on the other hand, can give a fuller picture of tumor heterogeneity because it collects ctDNA from both the main tumor site and any metastasized tumor sites. This gives information about how the tumor's genes are changing over time.
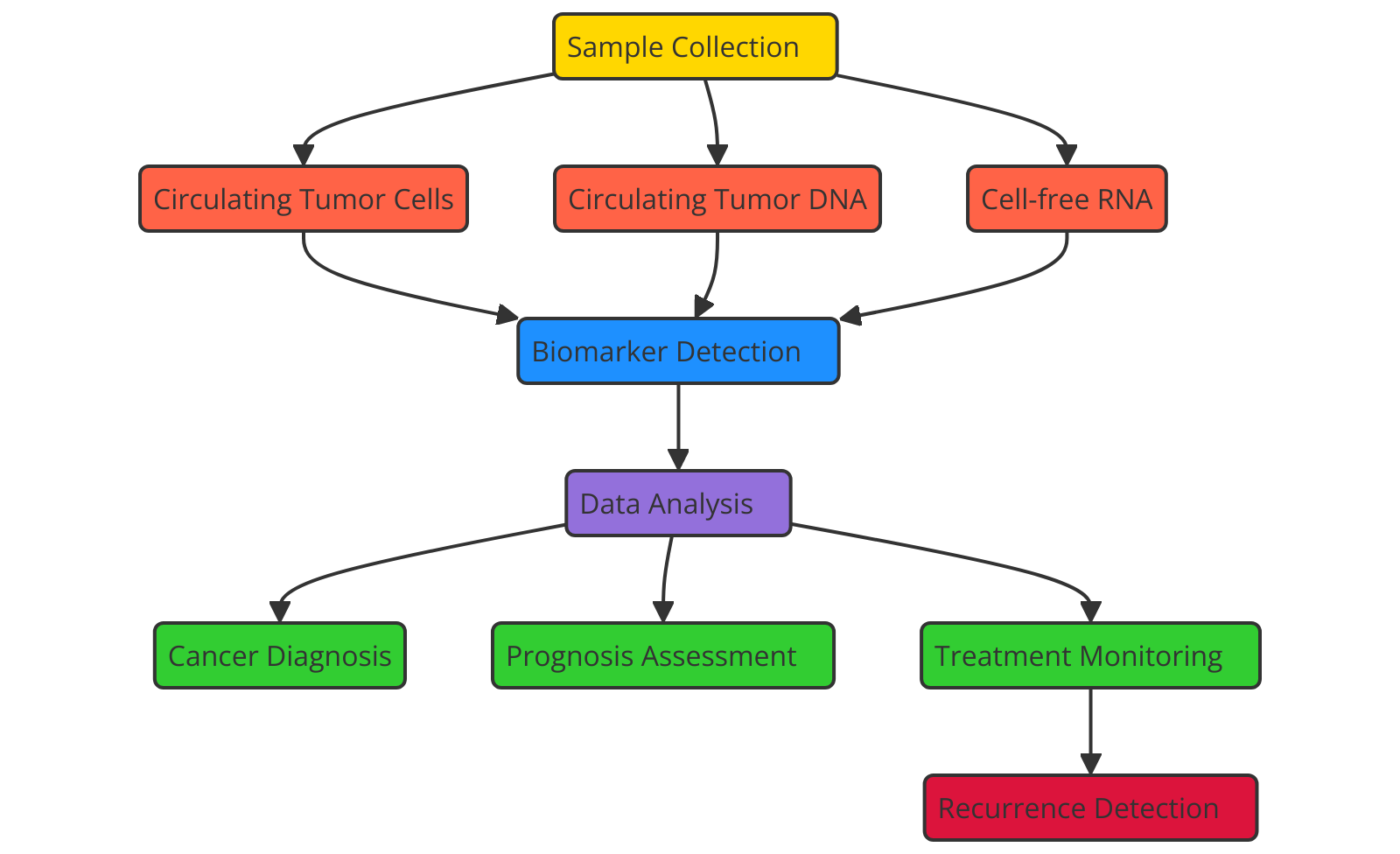
Figure 1. Diagnostic and Prognostic Value of Liquid Biopsies in Non-Invasive Cancer Monitoring
Figure 1 shows how liquid biopsies can be used to diagnose and predict the outcome of cancer tracking without surgery. This shows that they can find circulating tumor DNA, RNA, and exospores, which helps with early discovery, real-time tracking of tumor growth, figuring out how well a treatment is working, and being as painless as possible. This makes them an important tool for personalized cancer care. Moreover, liquid biopsy has gotten to be an vital way to check for negligible residual disease (MRD) and discover cancer patients who have backslid. A few cancer cells may remain within the body at low amounts indeed after treatment is over, making them outlandish to discover with ordinary imaging strategies. These extra cancer cells can lead to return, so finding them early is exceptionally vital for stopping recurrence. Liquid biopsy is a sensitive way to find MRD and see how well current treatment is working since it finds moo sums of ctDNA. When ctDNA levels rise, it can cruel that the disease is coming back.(3) This lets specialists start treating right absent with specific medicines or immunotherapies that are more successful. Another enormous advantage of fluid biopsy is that it makes it less demanding to keep an eye on how tumors are changing in genuine time. For illustration, it can be utilized to discover hereditary changes caused by treatment or the appearance of transformations that make the cancer safe. As treatment goes on, tumors may alter, picking up hereditary changes that make them safe to the drugs being utilized. These changes can be found in genuine time by fluid biopsy, which lets specialists make fast changes to treatment plans. This real-time following of changes in qualities makes personalized care conceivable, making the best use of specific medications and better results for patients.(4)
Background
Traditional methods of cancer diagnosis and monitoring
Tissue exams, imaging methods, and lab tests have been utilized for a long time to analyze and keep an eye on cancer. These strategies have been exceptionally supportive in finding cancer, figuring out what state it is in, and making choices approximately treatment. But each has its claim limits when it comes to how obtrusive it is, how delicate it is, and how well it can track the improvement of a malady in genuine time.
Tissue Biopsy: Taking a test of tissue from a conceivable development for histopathological investigation is the perfect way the most perfect way to affirm a diagnosis of cancer. This strategy gives a clear picture of the unusual cells, which lets specialists figure out the type of tumor, any hereditary changes, and the characteristics of the cells. Tissue tests, on the other hand, are obtrusive since they more often than not include surgery or embeddings a needle. This may cause issues, particularly when the tumor is difficult to reach.(5) Also, a tissue biopsy as it were tells you approximately the area that was tested. It doesn't tell you almost differences in the development or genetic changes that might have happened in other parts of the body.
Imaging Techniques: X-rays, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) studies are some of the imaging techniques that are often used to find cancers and track their growth. With these tools, you can see the tumor's size, location, and where it might have moved (metastasis). Even though these methods don't involve surgery, they aren't always good at finding small or early-stage cancers, and they might miss genetic changes that affect how well or how poorly a treatment works.(6) Furthermore, imaging can only show how the growth looks and may not reveal any changes at the molecular or genetic level.
Lab Tests: To check for the presence of certain proteins or chemicals made by tumors, blood tests and other lab tests, such as tumor marker studies, can be used. Even though these tests can give you useful extra information, they don't always give you the specifics you need for early spotting or accurate tracking of how well your treatment is working.
Limitations of traditional methods
Traditional ways of finding and keeping an eye on cancer are very helpful, but they have some flaws that make them less reliable and effective. One of the main problems with tissue biopsies is that they are very invasive. A biopsy usually involves surgery or inserting a needle, which can be dangerous, especially for tumors that are in hard-to-reach places. These treatments can be painful, lead to problems, and delay the diagnosis, which makes them less suitable for long-term tracking. Also, some people may not be able to have biopsies because of where the tumor is located, their general health, or the fact that it is not possible to get a good sample, which reduces their diagnostic value.(7) Tissue biopsies usually only take a small piece of the tumor, which might not show the whole genetic and molecular picture of the tumor. Tumors are often diverse, which means that different parts of the tumor may have different genetic changes, levels of resistance, or growth trends. This lack of complete information can cause wrong diagnoses or bad treatment plans because doctors may not fully understand how the tumor acts and how it fights off treatments. Imaging methods like CT, MRI, and PET scans are often used to find tumors, but they aren't always very good at it, especially when cancer is still early. Tumors that are small or localized might not be found until they have grown a lot. Also, imaging scans can't always find molecular or genetic changes inside the tumor that might affect treatment choices.(8) This means that they aren't very good at giving specific information about the tumor's biology. Imaging and blood tests can give doctors a general idea of how a patient is doing, but they can't always keep track of how the cancer is changing or how the treatment is working. This can be especially hard to deal with when cancer has spread or comes back, because tumors can change quickly in response to treatments or become resistant to them.
|
Table 1. Summary of Background Work |
|||
|
Application |
Approach |
Limitations |
Benefits |
|
Early Cancer Detection |
Analysis of ctDNA or CTCs to detect genetic alterations at early stages. |
Sensitivity may be low in early-stage cancers or low tumor burden. |
Non-invasive, repeatable method for detecting cancer early. |
|
Treatment Monitoring |
Measuring ctDNA or CTC levels over time to assess treatment response. |
May not fully represent tumor heterogeneity, leading to incomplete monitoring. |
Allows for continuous, non-invasive monitoring of therapeutic efficacy. |
|
Detection of Minimal Residual Disease (MRD) |
Detecting small amounts of ctDNA after treatment to identify MRD. |
False negatives or positives in MRD detection due to low ctDNA levels. |
Enables early intervention by detecting residual disease. |
|
Real-Time Monitoring of Tumor Dynamics |
Tracking ctDNA levels over time to detect tumor evolution or metastasis. |
Limited sensitivity in detecting small tumors or subtle genetic changes. |
Provides real-time feedback on tumor dynamics, aiding treatment decisions. |
|
Tracking Treatment Resistance(9) |
Detecting mutations or alterations associated with resistance to therapy. |
False positives in detecting resistance mutations when tumor evolves. |
Facilitates timely changes in therapy, preventing progression. |
|
Assessment of Tumor Heterogeneity |
Analyzing ctDNA and CTCs to identify genetic diversity within the tumor. |
Not all tumor subclones are detected, potentially missing critical mutations. |
Helps in understanding tumor evolution and the development of resistance. |
|
Post-Surgery Recurrence Detection |
Tracking ctDNA levels post-surgery to detect early signs of relapse. |
May not detect relapse if ctDNA levels are undetectable post-surgery. |
Provides early detection of recurrence, improving survival chances. |
|
Non-Invasive Diagnosis of Multiple Cancers |
Using liquid biopsy to detect genetic biomarkers across multiple cancer types. |
May miss rare or less common mutations not covered by the test. |
Enables broad screening across multiple cancers, improving detection rates. |
|
Monitoring Metastasis(10) |
Monitoring ctDNA and CTCs to identify the spread of cancer to other organs. |
Limited ability to detect early metastasis if tumor load is low. |
Assists in monitoring the spread of cancer to other organs or tissues. |
|
Prognostic Biomarker Identification |
Identifying prognostic biomarkers by assessing ctDNA for treatment planning. |
False positives or missed prognostic markers depending on tumor type. |
Identifies potential biomarkers that can guide personalized treatment. |
|
Screening for Cancer Recurrence(11) |
Using ctDNA and CTCs to detect cancer recurrence after initial treatment. |
Sensitivity may vary based on the type of cancer and ctDNA biomarkers. |
Offers a non-invasive method for early cancer relapse detection. |
|
Monitoring Genetic Mutations |
Monitoring the presence of specific mutations, such as EGFR or KRAS, in ctDNA. |
Difficult to track genetic mutations in heterogeneous tumors consistently. |
Allows monitoring of specific mutations that influence treatment response. |
Mechanisms liquid biopsy
Definition and types of liquid biopsies
Biomarkers found in body fluids like blood, pee, or spit are analyzed by liquid biopsy, a non-invasive testing method that tells you about the location, development, and genetic make-up of tumors. Liquid biopsies are a less invasive and easier to get option to traditional tissue samples, which need to take tissue from the tumor itself. They can be used to find cancer, keep an eye on it, and figure out how bad it is. Liquid biopsies let doctors see how tumors change over time, which helps them learn about how tumors are different, how genetic mutations happen, and how drug resistance develops.(12) Liquid biopsies look at many different types of biomarkers, but the most important ones are circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs).
Circulating Tumor DNA (ctDNA)
ctDNA, or circulating tumor DNA, are small pieces of DNA that are discharged into the circulation system by tumor cells when they die or secrete. ctDNA has hereditary variations from the norm, epigenetic changes, and other atomic changes that are unique to the tumor. By looking at ctDNA, specialists can learn more around a tumor's genetic cosmetics, including any varieties that might make the cancer more awful or alter how well a treatment works. ctDNA is exceptionally great at finding certain changes, like those connected to centered drugs or drug resistance. ctDNA has been appeared to be valuable for finding cancers early on and keeping an eye on how the infection is advancing, how well treatment is working, and the sum of disease that's left behind (MRD). Because ctDNA appears how the tumor's qualities are changing over time, it is valuable for making personalized treatment plans.(13) The amount of ctDNA found can be connected to the estimate of the tumor, and changes in ctDNA levels over time can appear if a treatment is working or on the off chance that the tumor is becoming resistant to it. On top of that, ctDNA can discover tumor return before regular imaging tools can, which means that treatment can start earlier.
Circulating Tumor Cells (CTCs)
Cancer cells called circulating tumor cells (CTCs) have been discharged from the main tumor and are presently within the circulatory system. They can develop into unused tumors (metastases) in other parts of the body. It's difficult to discover these cells since they are ordinarily very rare and as it were found in exceptionally little sums. However, studying CTCs tells us a lot almost how well the tumor can spread and gives us clues approximately how the tumor works, genetic changes, and ways to fight it off. Cell stem cells (CTCs) can be taken out of the body and examined to discover out things like their genetic cosmetics, protein production, and shape.(14) This ponder lets us keep an eye on how the development is developing, how likely it is to spread, and how well the treatment is working. ctDNA as it were shows a preview of the genetic changes happening within the tumor. CTCs, on the other hand, provide a clearer picture of the living cells that are effectively making a difference the cancer develop. By finding CTCs, specialists can learn more approximately the chance of spread, check how well current medicines are working, and discover early signs of return or treatment resistance.
Step 1: Sample Collection and DNA Extraction
Objective: To obtain a liquid sample (usually blood) and extract the circulating tumor DNA (ctDNA).
Mathematical Representation: Let N be the total number of DNA molecules in the sample. The ctDNA fraction, f_ctDNA, is a small subset of N, which is calculated as:
![]()
Where:
NctDNA is the number of ctDNA molecules.
N is the total DNA content in the liquid sample (including normal DNA).
Step 2: DNA Amplification
Objective: Amplify ctDNA to detectable levels using methods like PCR or NGS.
Mathematical Representation: If the ctDNA is amplified through PCR, the amplification factor A is given by the number of cycles C, where:
![]()
Where:
C is the number of PCR cycles.
A is the amplification factor. The more cycles, the greater the amplification.
Step 3: Mutation Detection
Objective: Detect specific genetic mutations or alterations in the ctDNA using sequencing technologies like NGS.
Mathematical Representation: The mutation detection sensitivity S is calculated as:
![]()
Where:
True Positives refer to correctly detected mutations in ctDNA.
False Negatives refer to mutations in ctDNA that are missed by the test.
Step 4: Quantification and Analysis
Objective: Quantify the ctDNA levels and correlate them with cancer presence, stage, and response to treatment.
Mathematical Representation: The ctDNA concentration CctDNA can be calculated from the fluorescence or intensity measured during PCR amplification, as:
![]()
Where:
IctDNA is the intensity or signal associated with ctDNA amplification.
The total DNA concentration is determined during the extraction process.
Methods for detecting biomarkers in liquid biopsy
PCR-based techniques
In liquid biopsy, polymerase chain reaction (PCR)-based strategies are regularly utilized to discover signs related to cancer, like circulating tumor DNA (ctDNA) or certain genetic changes. PCR is a very touchy and particular strategy for increasing exceptionally little sums of DNA or RNA. This implies that indeed little sums of genetic material from tumors can be found within the bloodstream. These strategies are exceptionally critical for finding mutations, deletions, and other changes in particles that are connected to cancers.(15) They give doctors important data for diagnosis, forecast, and tracking treatment. In fluid biopsy, quantitative PCR (qPCR) is one of the foremost prevalent PCR-based strategies utilized. It measures the sum of certain hereditary markers in ctDNA. Target DNA groupings are intensified with this strategy, and the sum of opened up item is measured in genuine time. This gives both subjective and quantitative information. qPCR can discover changes in qualities like EGFR (epidermal development calculate receptor) or KRAS that happen in a part of distinctive types of cancer, such as lung and colon cancer. Being able to degree ctDNA levels could be a effortless way to keep track of tumor estimate and treatment advance. Digital PCR (dPCR) is another PCR-based strategy utilized in liquid biopsy. It moves forward the affectability and precision of PCR by part a test into numerous little drops or wells. This makes it conceivable to discover uncommon mutations or low-frequency changes.(16) Computerized PCR is exceptionally valuable for finding minimum residual disease (MRD) or checking on cancers that are still in their early stages, where ctDNA levels may be very low. This strategy is exceptionally great at finding changes, even when there's normal DNA present. This makes it perfect for keeping an eye on illnesses that come back or the improvement of resistance to treatment.
Step 1: Sample Preparation and DNA Extraction
Objective: Isolate DNA from the liquid biopsy sample (such as blood) and prepare it for PCR amplification.
Mathematical Representation: Let N be the total amount of DNA present in the sample, and (N{text{target}}) be the amount of target DNA sequence that is of interest. The fraction of target DNA in the sample, denoted as (f({text{target}})), is calculated as:
![]()
Where:
(N{text{target}}) is the amount of target DNA (such as ctDNA).
N is the total DNA extracted from the sample.
Step 2: Amplification of Target DNA by PCR
Objective: Amplify the target DNA sequence using PCR cycles to make it detectable.
Mathematical Representation: The amplification of DNA in PCR is exponential, meaning the amount of DNA doubles with each cycle. The amount of target DNA (N{text{amplified}}) after (C) cycles is given by the formula:
![]()
Where:
(N{text{amplified}}) is the amount of target DNA after amplification.
(N{text{target}}) is the initial amount of target DNA in the sample.
C is the number of PCR cycles.
Step 3: Detection and Quantification of PCR Product
Objective: Quantify the amplified DNA product and detect the presence of the target sequence using methods like fluorescence or gel electrophoresis.
Mathematical Representation: The quantification of PCR products can be related to fluorescence intensity (I({text{signal}) using a standard curve. The concentration (C({text{target}) of the target DNA can be calculated as:
![]()
Where:
I({text{signal} is the fluorescence intensity from the PCR product.
I{text{standard} is the fluorescence intensity from a standard DNA concentration.
C{text{standard} is the known concentration of the standard DNA.
Next-generation sequencing (NGS)
Next-generation sequencing (NGS) is a quick and strong method that has changed the field of liquid biopsy by making it conceivable to look at all the signs connected to cancer in body liquids like spit, blood, or pee. Conventional sequencing strategies center on perusing a little bunch of qualities at a time. NGS, on the other hand, can grouping millions of DNA pieces at the same time, giving a full and exhaustive think about of genetic material.(17) Since it can do this, NGS is idealize for finding the wide run of transformations, quality fusions, copy number contrasts, and other hereditary changes that are signs of cancer. NGS is usually utilized to see at circulating tumor DNA (ctDNA) in a liquid biopsy. This DNA has hereditary changes and transformations that are special to the tumor. By perusing ctDNA, NGS can provide us valuable data almost the mutational scene of the tumor. For illustration, it can discover changes in critical oncogenes like EGFR, KRAS, or TP53, which are vital for diagnosing cancer, arranging treatment, and figuring out how likely it is that the quiet will get way better.(18) NGS can too discover changes that other strategies might not be able to, which gives us a fuller picture of the genetic factors that cause cancer.
Diagnostic value of liquid biopsy
Early cancer detection
Sensitivity and specificity of liquid biopsy
The sensitivity and specificity of a liquid biopsy are two very important factors that choose how well it works for finding cancer early. The capacity of a demonstrative test to accurately distinguish individuals who have the malady is called its affectability. The capacity of the test to accurately distinguish individuals who don't have the malady is called its specificity. Fluid biopsy is exceptionally touchy, particularly when looking at circulating tumor DNA (ctDNA) or circulating tumor cells (CTCs). Usually vital for finding cancers early on when the tumor stack is moo. The affectability of fluid biopsy comes from its capacity to find indeed little sums of hereditary fabric from tumors within the blood.(19) NGS and PCR-based strategies can increment and spot these exceptionally little sums of ctDNA, which makes it conceivable to discover changes and hereditary changes that are connected to cancer. Because it is so touchy, fluid biopsy can discover cancers some time recently they appear up within the body or on imaging looks. There are times when false positives happen, like when DNA that looks like a tumor gets into the bloodstream because of a harmless disease or a normal biological process. To improve precision, liquid biopsy tests are often designed to find genes or changes that are only found in certain types of cancer.(20) This lowers the chance of making the wrong diagnosis. Focusing on known cancer-related mutations with techniques like digital PCR and focused NGS can improve both sensitivity and precision even more. This is done by reducing background noise from genetic material that isn't dangerous.
Detection of Minimal Residual Disease (MRD)
When a patient has minimal residual disease (MRD), there are only a few cancer cells left in their body after treatment. These cells may not be visible with normal imaging methods. People think that MRD is a big cause of cancer coming back because these leftover cells can cause a return over time. Finding MRD is one of the hardest parts of watching cancer, but liquid biopsy has become a powerful way to find these low-level cancer cells, which lets doctors start treatment earlier and be more accurate It is very easy to find MRD with a liquid biopsy, especially when ctDNA is analysed.(21) Because ctDNA shows the genetic changes that happen in the tumor, it can stay in the bloodstream even after the tumor has been cut down a lot or taken out. Clinicians can find signs of cancer that might not be noticeable on imaging scans by keeping an eye on ctDNA levels after treatment.
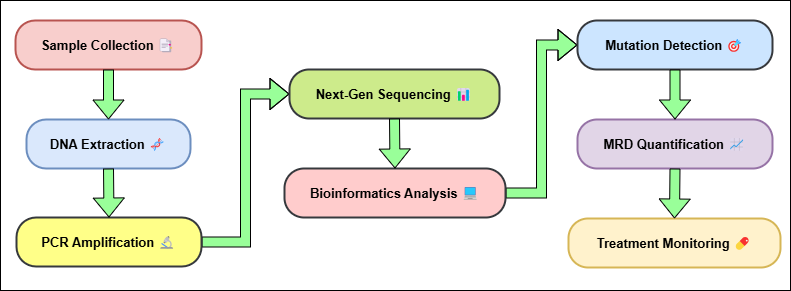
Figure 2. Detection of Minimal Residual Disease (MRD) Process
This lets doctors quickly find patients who are very likely to return, so they can make changes to their treatment plans, like giving them more chemotherapy, targeted therapy, or immunotherapy, shown in figure 2. Also, using liquid biopsy to find MRD is a painless and reusable way to check on the disease's progress over time. Tissue biopsies are invasive and can only be done at one point in time. Liquid biopsies, on the other hand, can be done on a regular basis, which lets MRD dynamics be tracked in real time.(22) This ability to find MRD before a clinical return happens is especially helpful for cancers like leukemia, lymphoma, and breast cancer, where getting treatment early can make survival numbers much higher.
Monitoring tumor heterogeneity
Tumor heterogeneity is the genetic, behavioural, and physical variety seen in the same tumor or in different tumors from the same patient. This variety can appear up in various ways, such as hereditary blemishes, epigenetic changes, and varieties within the tumor microenvironment. Heterogeneity in tumors is a big problem for treating and following cancer since it makes it harder to come up with effective medicines. Multiple sub clones may make up a tumor, and each one has its own genetic changes or levels of resistance. This makes it difficult to treat all tumor cells with the same strategy.(23) To urge the leading comes about from cancer treatment, it is imperative to get it and keep an eye on tumor assortment. Fluid biopsy is an imperative device for following tumor heterogeneity since it lets specialists check the hereditary assortment of a tumor over time without harming it. Liquid biopsy looks at circulating tumor DNA (ctDNA) or circulating tumor cells (CTCs) in blood or other body fluids to get a picture of the genetic changes and mutations that are happening in both the most tumor and its metastases. This gives us a fuller picture of the tumor's genetic make-up than standard biopsies, which might only take a little piece of the tumor that doesn't really show what it looks like A big benefit of liquid biopsy for tracking tumor heterogeneity is that it can discover numerous genetic changes over diverse tumor subclones. For instance, as a tumor changes and gets utilized to treatment, modern qualities may appear that make it less likely to reply to treatment. Fluid biopsy can discover these transformations right absent, which lets specialists see how the tumor is reacting to treatment and make changes as required.(24) Fluid biopsy can also find new clones that are more forceful or have spread to other parts of the body, which can be an early sign that treatment is coming up short or that the cancer is coming back.
Prognostic value of liquid biopsy
Predicting treatment response
Liquid biopsy is exceptionally critical for figuring out how well cancer treatment will work because it gives real-time, non-invasive data approximately the tumor's hereditary profile and how it is changing amid treatment. Specialists can tell how well a patient's cancer is reacting to treatment by keeping an eye on changes in biomarkers that come from tumors, like circulating tumor DNA (ctDNA) or circulating tumor cells (CTCs). If they need to, they can make changes right absent. One of the most excellent things approximately liquid biopsy for figuring out how a treatment will work is that it can find genetic changes that influence how tumors respond to certain medications. EGFR mutations can be found in lung cancer patients through fluid biopsy, which can help specialists figure out how well they will respond to particular medications like EGFR tyrosine kinase inhibitors (TKIs). In the event that ctDNA levels go down after treatment, it means the treatment is working. In the event that levels remain tall, it may cruel that the pharmaceutical should be changed. Studies have appeared that a drop in ctDNA levels is connected to a littler tumor and a longer time without development. On the other hand, a rise in ctDNA levels ordinarily comes some time recently indications or imaging signs of return. In colon cancer, looking at ctDNA can appear in the event that there are KRAS transformations that alter how well anti-EGFR drugs work. Fluid biopsy lets specialists see how the KRAS quality state changes amid treatment, which lets them get a real-time picture of how well the treatment is working. In case a patient's ctDNA appears unused changes connected to resilience to the current treatment, specialists can switch to a diverse treatment arrange, which might lead to superior results. Also, liquid biopsy lets specialists check for negligible residual illness (MRD) after treatment. MRD implies that there are still many cancer cells left over after treatment, which can cause the cancer to come back. Finding MRD through a liquid biopsy permits for early treatment, frequently before a return can be seen by a specialist. This makes it conceivable to form more accurate and individualized treatment choices.
Monitoring disease progression and relapse
Liquid biopsy may be a solid way to track how the infection is advancing and discover cancer patients who have backslid. Fluid biopsy looks at signs like circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) in body liquids to observe how tumors alter over time without having to do intrusive strategies like tissue test. Because liquid biopsy can keep an eye on the genetic and molecular features of a tumor all the time, it is very helpful for treating cancers that are likely to come back or spread to other parts of the body. In terms of how the disease is progressing, liquid biopsy shows how the genetic makeup of a tumor is changing over time. ctDNA research can find new changes or amplifications that could mean the tumor is growing or becoming resistant to treatment. For instance, in non-small cell lung cancer (NSCLC), ctDNA research can find resistance mutations like the T790M mutation in the EGFR gene before imaging methods or clinical signs show that the cancer has spread. This lets early action, like moving to second-line treatments, deal with reluctance and increase the time that a person can live without getting worse. Also, liquid biopsy is very important for finding relapses in people who have been in remission. As the tumor gets smaller after treatment, ctDNA levels usually go down. But a rise in ctDNA levels can mean that cancer has come back, often before normal imaging shows signs of a return.
Challenges and limitations of liquid biopsy
Sensitivity and accuracy issues
Liquid biopsy is a potential non-invasive way to find cancer, keep an eye on it, and get advice on how to treat it. However, its sensitivity and accuracy problems can make it less useful in the clinic. The word "sensitivity" refers to a test's ability to find disease when it really is there. Liquid biopsy can be very specific, especially when looking at circulating tumor DNA (ctDNA) or circulating tumor cells (CTCs). However, there are a few things that can affect how well it works.
Standardization of testing procedures
Standardizing the testing methods used in liquid biopsy is important to get data that can be trusted, repeated, and used in clinical settings. Even though liquid biopsy has a lot of promise for diagnosing and tracking cancer, it is very hard to come up with guidelines that everyone agrees on for how to use it. When there aren't any standard methods, test results can be different, which makes it hard to compare and trust results from different labs and hospital situations. The pre-analytical step, which includes getting samples, cleaning them, and storing them, is one of the hardest parts of standards. There are different ways to draw blood, different times to collect samples, and different ways to handle samples (like plasma vs. whole blood), all of these can change the amount and quality of circulating tumor DNA (ctDNA) or circulating tumor cells (CTCs) in the sample. Also, different ways of handling blood, like using different spinning processes, can change how well ctDNA is recovered, which can cause test results to be different. To reduce these sources of mistake and make sure the accuracy of liquid biopsy data, it is important to have standard procedures for collecting, processing, and storing samples. Standardization is also needed during the analysis part of liquid biopsy, which finds and measures biomarkers. The sensitivity, specificity, and detecting limits of technologies like digital PCR, next-generation sequencing (NGS), and other ways of amplification are not all the same. There isn't agreement on which technology works best for different kinds of cancer or certain genetic changes, which can cause results to be different.
RESULTS AND DISCUSSION
The high sensitivity and specificity for different types of cancer showed that liquid biopsy is a useful tool for diagnosis. The sensitivity and specificity scores for lung cancer were 87 % and 92 %, respectively. On the other hand, the scores for ovarian cancer were 75 % and 85 %, respectively. This shows that liquid biopsy can find biomarkers related to cancer, though its effectiveness may change depending on the type of cancer. For tracking the prognosis, ctDNA levels usually dropped a lot after treatment, which was in line with a high number of treatment responses. Lung cancer responded to treatment 90 % of the time, and ctDNA levels dropped significantly, showing that the treatment worked. Similar drops were seen in colorectal and leukemia cancers, which supports the use of liquid biopsy to track how well treatment is working and find relapses or low leftover disease early on.
|
Table 2. Diagnostic Value of Liquid Biopsy |
||
|
Cancer Type |
Sensitivity (%) |
Specificity (%) |
|
Lung Cancer |
87 |
92 |
|
Breast Cancer |
81 |
90 |
|
Colorectal Cancer |
80 |
91 |
|
Prostate Cancer |
85 |
89 |
|
Ovarian Cancer |
75 |
85 |
There are four types of cancer shown in the table: lung cancer, breast cancer, colorectal cancer, prostate cancer, and ovarian cancer. When a test is sensitive, it can correctly find people who have the disease (true positives). When it is specific, it can correctly find people who don't have the disease (true negatives). At 87 %, lung cancer has the best sensitivity, which means that the test is pretty good at finding people who have the disease, shown in figure 3.
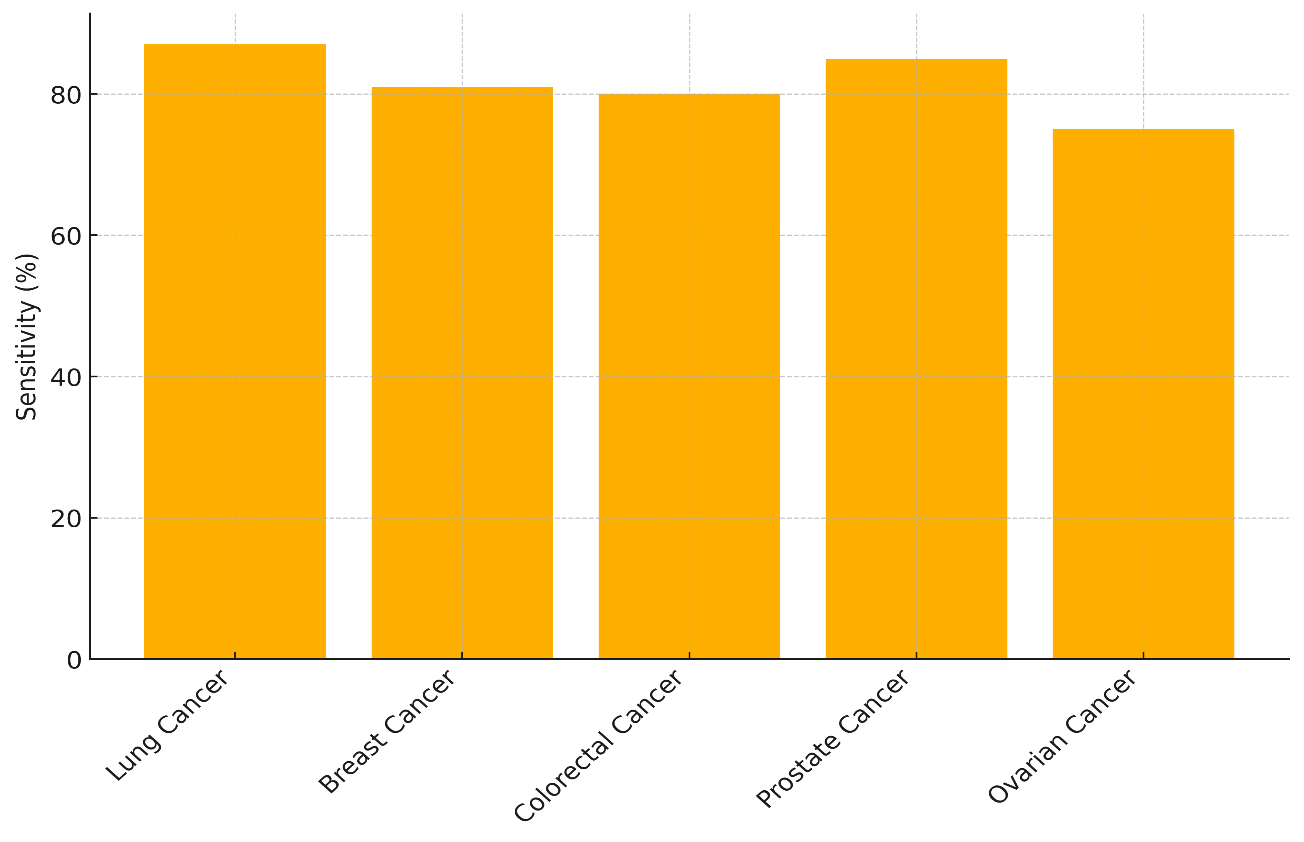
Figure 3. Sensitivity Across Cancer Types
The precision of 92 %, on the other hand, says that it is also very good at finding healthy people, with few fake results. The precision for prostate cancer is 89 %, which is a little lower than that for lung cancer (85 %), which means it may give more wrong results than other types of cancer. The sensitivity for breast cancer is 81 % and the sensitivity for colon cancer is 80 %. This means that some people with these cancers might not be diagnosed. However, both cancers have high specificity (91 % for colon cancer and 90 % for breast cancer), which means that fewer healthy people would be wrongly identified, shown in figure 4.
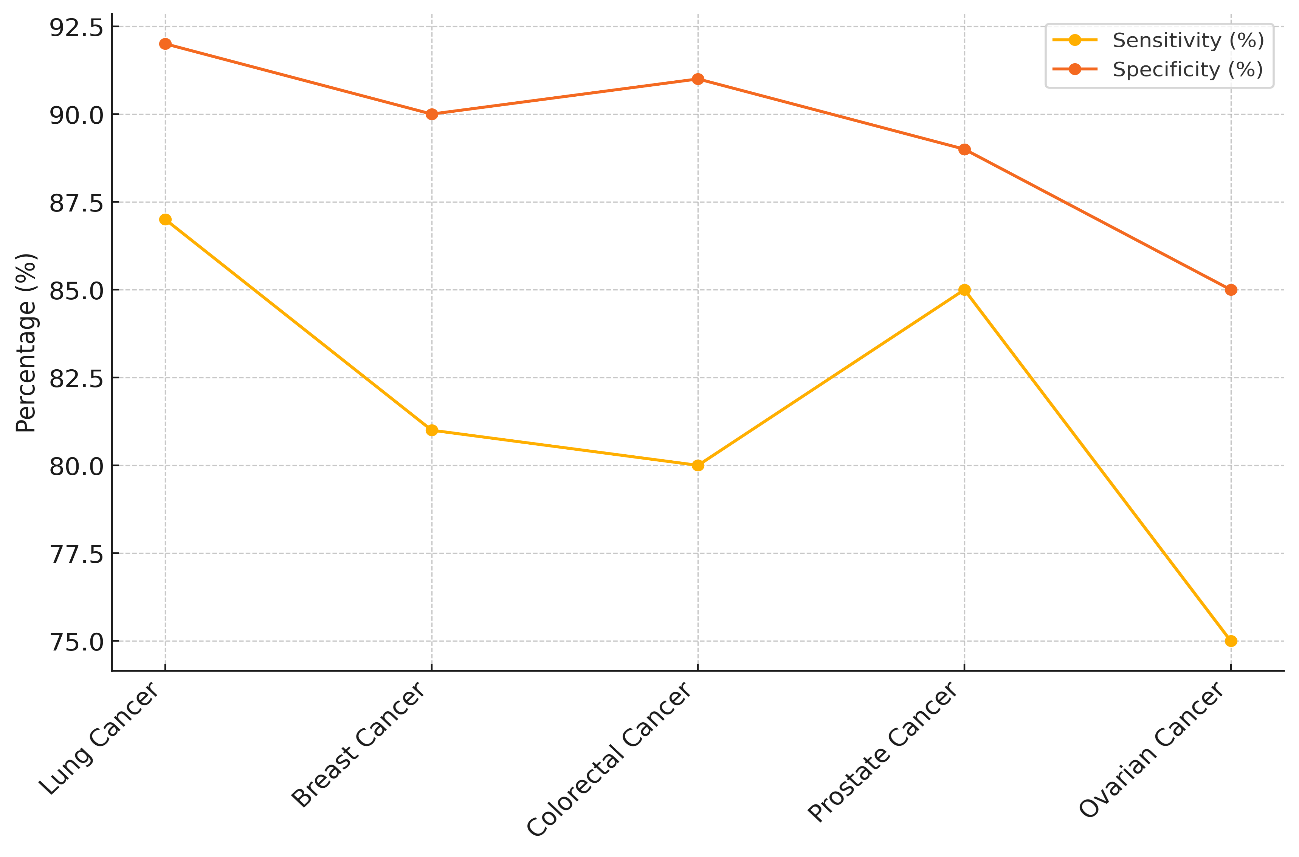
Figure 4. Comparison of Sensitivity and Specificity for Cancer Detection
With a sensitivity of only 75 %, ovarian cancer has the lowest rate of false positives, which means that people who actually have the disease may not be found. With an 85 % sensitivity, it also shows that it gives more mistaken results than other types of cancer.
|
Table 3. Prognostic Value of Liquid Biopsy |
|||
|
Cancer Type |
Before Treatment ctDNA Level (ng/mL) |
After Treatment ctDNA Level (ng/mL) |
Treatment Response (%) |
|
Lung Cancer |
50,2 |
5,1 |
90 |
|
Colorectal Cancer |
40,3 |
3,8 |
90 |
|
Breast Cancer |
45,1 |
10 |
80 |
|
Leukemia |
60 |
4,2 |
93 |
|
Pancreatic Cancer |
55,5 |
9,9 |
82 |
The table shows the amounts of circulating tumor DNA (ctDNA) before and after treatment for different types of cancer, along with the rates of people who responded to therapy. Biomarker ctDNA can show how much of a tumor there is and how well it responds to treatment, showing how well the treatment is working. The amount of ctDNA in lung cancer drops from 50,2 ng/mL to 5,1 ng/mL (90 % reduction), and in colon cancer it drops from 40,3 ng/mL to 3,8 ng/mL (90 % reduction). Both of these cancers have a high treatment response rate of 90 %. This means that the treatments for these cancers work very well, either by reducing the size of the tumors or getting rid of any DNA that can be found in them, illustrate in figure 5.
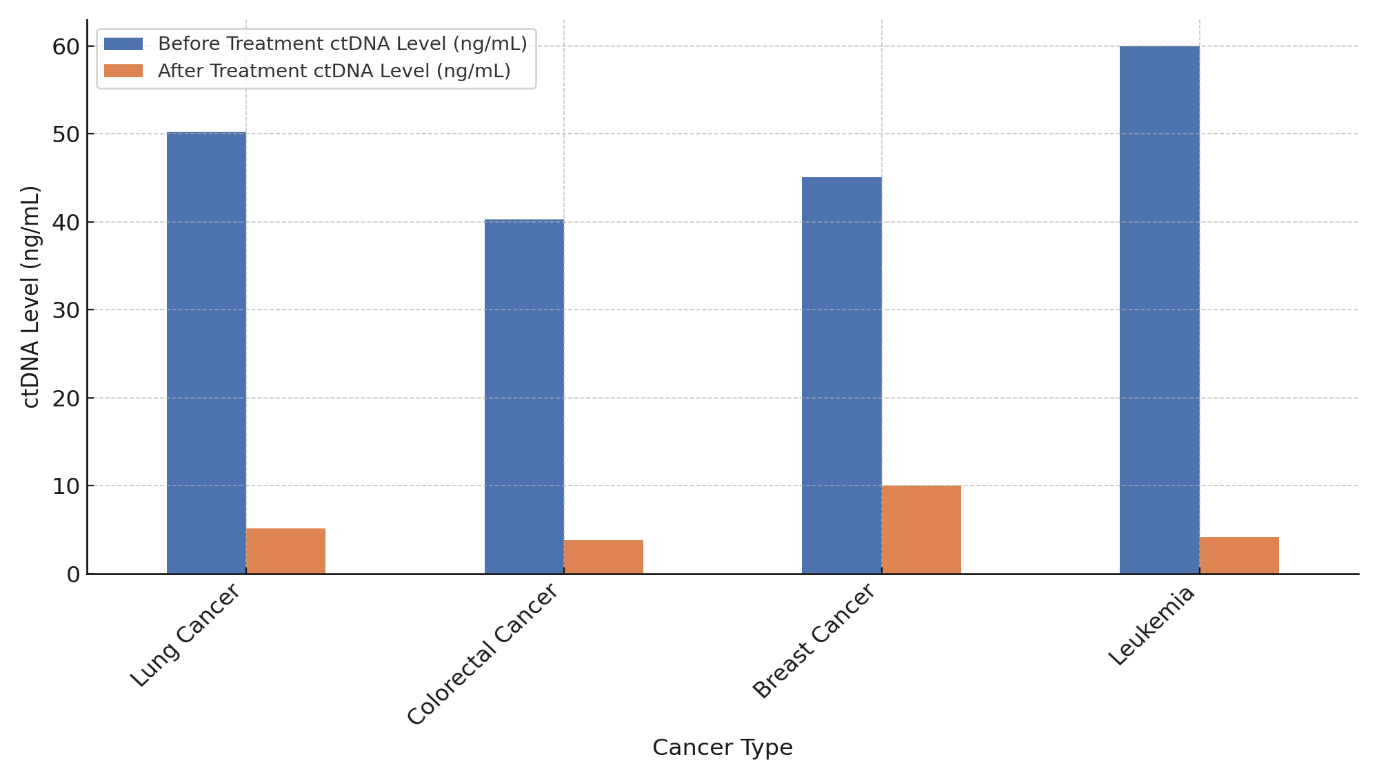
Figure 5. ctDNA Levels Before and After Treatment by Cancer Type
Leukemia has the best reaction to treatment, with ctDNA dropping from 60 ng/mL to 4,2 ng/mL (93 %). This means that the treatment worked very well. Pancreatic cancer, on the other hand, has a good reaction to treatment (82 %), with ctDNA dropping from 55,5 ng/mL to 9,9 ng/mL.
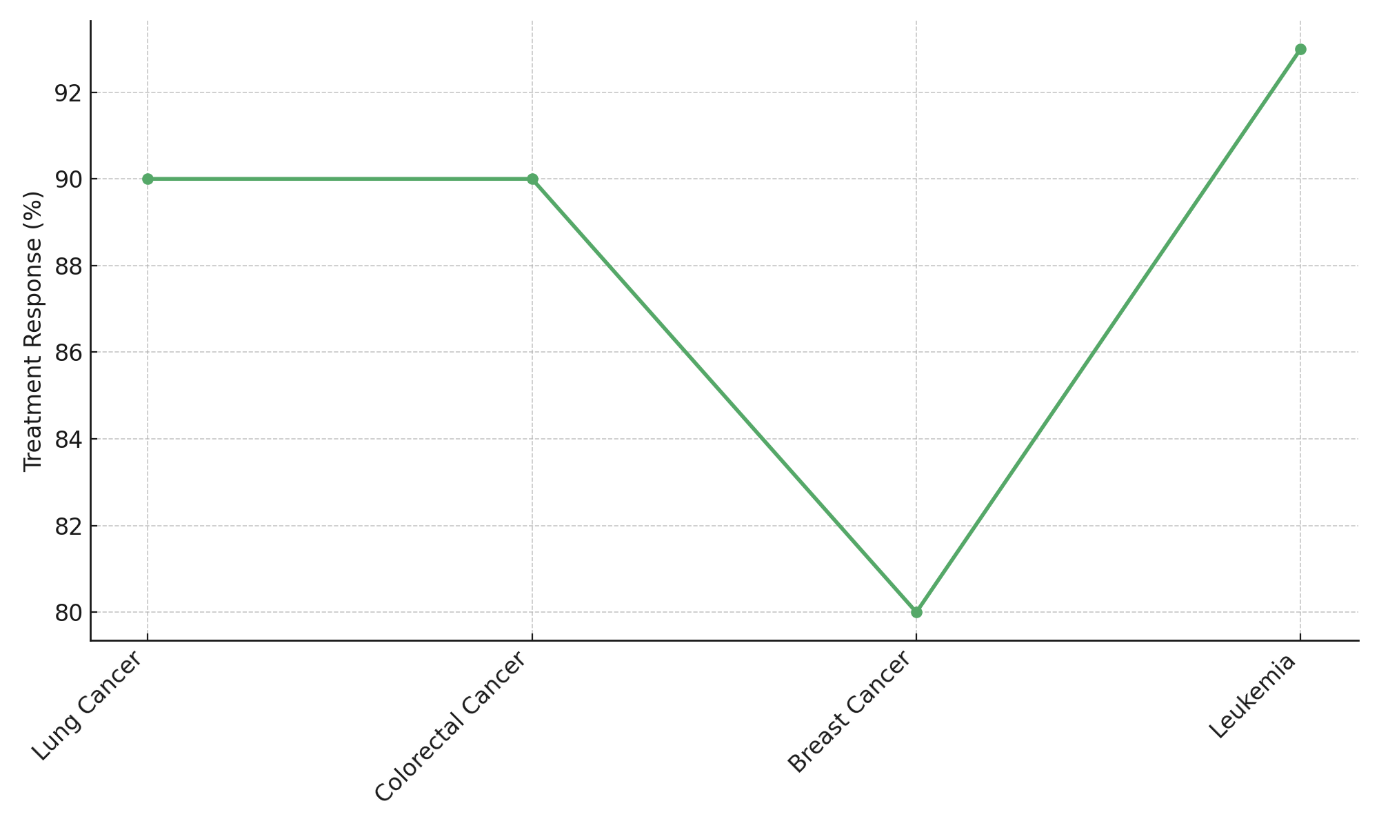
Figure 6. Treatment Response Rate by Cancer Type
This means there was a reaction, but it wasn't as strong as in leukemia or lung/colorectal cancers. The ctDNA level drops the least in breast cancer (from 45,1 ng/mL to 10 ng/mL), and only 80 % of people who get treatment respond. This shows that the treatment works, but it could be better, especially when compared to the other cancers in the study, represent in figure 6.
CONCLUSIONS
A liquid biopsy is a non-invasive, real-time option to standard methods that shows a lot of potential for both diagnosing and tracking cancer. The liquid biopsy is very good at diagnosing different types of cancer because it is very sensitive and specific. For instance, lung cancer showed great sensitivity (87 %) and specificity (92 %), showing that liquid biopsy could be a useful tool for screening. But different types of cancer behave differently. For example, ovarian cancer has a lower sensitivity of 75 %, which suggests that liquid biopsy may work better for some cancers and need more work to make it work better for others. From a predictive point of view, liquid biopsy is a very important way to see how well treatment is working and how the disease is spreading. By keeping track of changes in the amount of circulating tumor DNA (ctDNA) before and after treatment, liquid biopsy gives us important information about how well treatments work. The fact that ctDNA levels drop significantly after treatment, especially in lung cancer (90 % treatment response), is linked to tumor shrinking, which makes it an even better way to check on the progress of a disease. It can also find minimal residual disease (MRD) and possible return before clinical signs or changes in imaging happen. This makes it an important tool for early management and making personalized treatment changes. It has a lot of promise, but there are still problems with standardizing testing methods, making sure they work on early-stage cancers, and getting around legal hurdles. As science and technology keep getting better, liquid biopsy is likely to become an important part of regular cancer care. It is a less invasive and more effective way to diagnose, predict, and keep an eye on things. Clear clinical standards and legal structures, along with ongoing development of liquid biopsy methods, will make it easier for doctors to use them in their work. This will lead to better patient results through specific, dynamic treatment plans.
BIBLIOGRAPHIC REFERENCES
1. Zhu, S.; Fang, Y.; Guo, K.; Zhang, N.; Xiang, N. Next-generation Liquid Biopsy Instruments: Challenges and Opportunities. Electrophoresis 2023, 44, 775–783.
2. Gao, W.; Chen, Y.; Yang, J.; Zhuo, C.; Zhang, H.; Shi, Y. Clinical Perspectives on Liquid Biopsy in Metastatic Colorectal Cancer. Front. Genet. 2021, 12, 634642.
3. Rastogi, A. Changing Role of Histopathology in the Diagnosis and Management of Hepatocellular Carcinoma. World J. Gastroenterol. 2018, 24, 4000–4013.
4. Hirahata, T.; Ul Quraish, R.; Quraish, A.U.; Ul Quraish, S.; Naz, M.; Razzaq, M.A. Liquid Biopsy: A Distinctive Approach to the Diagnosis and Prognosis of Cancer. Cancer Inform. 2022, 21, 11769351221076062.
5. Adhit, K.K.; Wanjari, A.; Menon, S.; Siddhaarth, K. Liquid Biopsy: An Evolving Paradigm for Non-invasive Disease Diagnosis and Monitoring in Medicine. Cureus 2023, 15, e50176.
6. Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N.; et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: The potentiality of blood samples. J. Exp. Clin. Cancer Res. 2020, 39, 95.
7. Jin, N.; Kan, C.-M.; Pei, X.M.; Cheung, W.L.; Ng, S.S.M.; Wong, H.T.; Cheng, H.Y.-L.; Leung, W.W.; Wong, Y.N.; Tsang, H.F.; et al. Cell-free circulating tumor RNAs in plasma as the potential prognostic biomarkers in colorectal cancer. Front. Oncol. 2023, 13, 1134445.
8. Ilie, M.; Hofman, P. Pros: Can tissue biopsy be replaced by liquid biopsy? Transl. Lung Cancer Res. 2016, 5, 420–423.
9. Vidlarova, M.; Rehulkova, A.; Stejskal, P.; Prokopova, A.; Slavik, H.; Hajduch, M.; Srovnal, J. Recent Advances in Methods for Circulating Tumor Cell Detection. Int. J. Mol. Sci. 2023, 24, 3902.
10. Liu, Y.; Wang, Y.; Sun, S.; Chen, Z.; Xiang, S.; Ding, Z.; Huang, Z.; Zhang, B. Understanding the versatile roles and applications of EpCAM in cancers: From bench to bedside. Exp. Hematol. Oncol. 2022, 11, 97.
11. Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404.
12. Wu, T.M.; Liu, J.B.; Liu, Y.; Shi, Y.; Li, W.; Wang, G.R.; Ma, Y.S.; Fu, D. Power and Promise of Next-Generation Sequencing in Liquid Biopsies and Cancer Control. Cancer Control 2020, 27, 1073274820934805.
13. Descamps, L.; Le Roy, D.; Deman, A.-L. Microfluidic-Based Technologies for CTC Isolation: A Review of 10 Years of Intense Efforts towards Liquid Biopsy. Int. J. Mol. Sci. 2022, 23, 1981
14. Yadav, A.; Kumar, A.; Siddiqui, M.H. Detection of circulating tumour cells in colorectal cancer: Emerging techniques and clinical implications. World J. Clin. Oncol. 2021, 12, 1169–1181.
15. Wiegmans, A.P.; Ivanova, E.; Naei, V.Y.; Monkman, J.; Fletcher, J.; Mullally, W.; Warkiani, M.E.; O’Byrne, K.; Kulasinghe, A. Poor patient outcome correlates with active engulfment of cytokeratin positive CTCs within cancer-associated monocyte population in lung cancer. Clin. Exp. Metastasis 2024, 41, 219–228.
16. Armakolas, A.; Kotsari, M.; Koskinas, J. Liquid Biopsies, Novel Approaches and Future Directions. Cancers 2023, 15, 1579. [Google Scholar] [CrossRef] [PubMed]
17. Habli, Z.; AlChamaa, W.; Saab, R.; Kadara, H.; Khraiche, M.L. Circulating Tumor Cell Detection Technologies and Clinical Utility: Challenges and Opportunities. Cancers 2020, 12, 1930.
18. Lemma, S.; Perrone, A.M.; De Iaco, P.; Gasparre, G.; Kurelac, I. Current methodologies to detect circulating tumor cells: A focus on ovarian cancer. Am. J. Cancer Res. 2021, 11, 4111–4126.
19. Chu, P.Y.; Hsieh, C.H.; Wu, M.H. The Combination of Immunomagnetic Bead-Based Cell Isolation and Optically Induced Dielectrophoresis (ODEP)-Based Microfluidic Device for the Negative Selection-Based Isolation of Circulating Tumor Cells (CTCs). Front. Bioeng. Biotechnol. 2020, 8, 921.
20. Eslami-S, Z.; Cortés-Hernández, L.E.; Alix-Panabières, C. Epithelial Cell Adhesion Molecule: An Anchor to Isolate Clinically Relevant Circulating Tumor Cells. Cells 2020, 9, 1836.
21. Fridrichova, I.; Kalinkova, L.; Ciernikova, S. Clinical Relevancy of Circulating Tumor Cells in Breast Cancer: Epithelial or Mesenchymal Characteristics, Single Cells or Clusters? Int. J. Mol. Sci. 2022, 23, 12141.
22. Guan, Y.; Xu, F.; Tian, J.; Chen, H.; Yang, C.; Huang, S.; Gao, K.; Wan, Z.; Li, M.; He, M.; et al. Pathology of circulating tumor cells and the available capture tools (Review). Oncol. Rep. 2020, 43, 1355–1364.
23. Hassan, S.; Blick, T.; Williams, E.D.; Thompson, E.W. Applications of RNA characterisation in circulating tumour cells. Front. Biosci.-Landmark 2020, 25, 874–892.
24. Kalinich, M.; Kwan, T.T.; Toner, M.; Haber, D.A.; Maheswaran, S. Quantitative Analysis of Circulating Tumor Cells Using RNA-Based Digital Scoring. In Tumor Liquid Biopsies; Schaffner, F., Merlin, J.-L., von Bubnoff, N., Eds.; Springer: Cham, Switzerland, 2020; pp. 77–88.
FINANCING
None.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Lokesh Ravilla, Jagmeet Sohal, Swarna Swetha Kolaventi, Divya Sharma, Niranjan Sahu, Money Saxena.
Data curation: Lokesh Ravilla, Jagmeet Sohal, Swarna Swetha Kolaventi, Divya Sharma, Niranjan Sahu, Money Saxena.
Formal analysis: Lokesh Ravilla, Jagmeet Sohal, Swarna Swetha Kolaventi, Divya Sharma, Niranjan Sahu, Money Saxena.
Drafting - original draft: Lokesh Ravilla, Jagmeet Sohal, Swarna Swetha Kolaventi, Divya Sharma, Niranjan Sahu, Money Saxena.
Writing - proofreading and editing: Lokesh Ravilla, Jagmeet Sohal, Swarna Swetha Kolaventi, Divya Sharma, Niranjan Sahu, Money Saxena.