doi: 10.56294/mw2024551
ORIGINAL
X-Ray Absorption Spectroscopy: A Comprehensive Overview
Espectroscopia de absorción de rayos X: Una visión completa
Rakesh Kumar Ranjan1 *
1Department of Physics, S. B. College. Ara, India.
Cite as: Ranjan RK. X-Ray Absorption Spectroscopy: A Comprehensive Overview. Seminars in Medical Writing and Education. 2024; 3:551. https://doi.org/10.56294/mw2024551
Submitted: 17-11-2023 Revised: 06-02-2024 Accepted: 24-04-2024 Published: 25-04-2024
Editor: PhD.
Prof. Estela Morales Peralta ![]()
Corresponding Author: Rakesh Kumar Ranjan *
ABSTRACT
Introduction: X-Ray Absorption Spectroscopy (XAS) is a powerful analytical tool that provides critical insights into the electronic and structural characteristics of elements in various samples.
Objective: widely employed across disciplines such as materials science, chemistry, biology, and physics, XAS enables researchers to study atomic-scale properties, including chemical states, local bonding environments, and coordination structures. By analyzing the energy-dependent absorption of X-rays, this technique delivers a detailed understanding of the fundamental interactions within complex systems.
Method: this paper presents an in-depth exploration of the principles and methodologies underlying XAS, focusing on its experimental design and data analysis strategies.
Results: it highlights the broad applicability of XAS, ranging from investigating catalytic materials and energy storage systems to analyzing biological processes and environmental contaminants. Recent technological advancements, including enhanced synchrotron radiation sources, improved computational tools, and the development of time-resolved XAS methods, have expanded its capabilities, making it more precise and adaptable to modern research challenges.
Conclusions: the study emphasizes the growing importance of XAS in uncovering the intricacies of diverse systems and addresses future trends in the field. By showcasing the innovative potential of this technique, it reinforces its pivotal role in advancing knowledge across scientific disciplines while paving the way for further methodological and application-oriented breakthroughs.
Keywords: X-Ray Absorption Spectroscopy; Solid State Physics; Photoelectric Effect.
RESUMEN
Introducción: la Espectroscopía de Absorción de Rayos X (XAS) es una poderosa herramienta analítica que proporciona conocimientos críticos sobre las características electrónicas y estructurales de los elementos en diversas muestras.
Objetivo: la XAS, ampliamente utilizada en disciplinas como la ciencia de los materiales, la química, la biología y la física, permite a los investigadores estudiar las propiedades a escala atómica, incluidos los estados químicos, los entornos de enlace locales y las estructuras de coordinación. Mediante el análisis de la absorción de rayos X en función de la energía, esta técnica permite comprender en detalle las interacciones fundamentales dentro de sistemas complejos.
Método: este artículo presenta una exploración en profundidad de los principios y metodologías subyacentes a XAS, centrándose en su diseño experimental y en las estrategias de análisis de datos.
Resultados: destaca la amplia aplicabilidad del XAS, que abarca desde la investigación de materiales catalíticos y sistemas de almacenamiento de energía hasta el análisis de procesos biológicos y contaminantes medioambientales. Los recientes avances tecnológicos, como las fuentes de radiación sincrotrón mejoradas,
las herramientas computacionales perfeccionadas y el desarrollo de métodos de XAS resueltos en el tiempo, han ampliado sus capacidades, haciéndolo más preciso y adaptable a los retos de la investigación moderna.
Conclusiones: el estudio subraya la creciente importancia de la XAS para descubrir las complejidades de diversos sistemas y aborda las tendencias futuras en este campo. Al mostrar el potencial innovador de esta técnica, refuerza su papel fundamental en el avance de los conocimientos en todas las disciplinas científicas, al tiempo que allana el camino para nuevos avances metodológicos y orientados a la aplicación.
Palabras clave: Espectroscopia de Absorción de Rayos X; Física del Estado Sólido; Efecto Fotoeléctrico.
INTRODUCTION
Now a days XAS (X-Ray Absorption spectroscopy) has become a powerful tool for proving the local structure and related Physico- chemical properties in different fields of study, e.g. in plasma physics, Astrophysics, solid state Physics, Chemical Physics, atomic and catalysts and surface studies, phase transition and dilute solutions, metals alloys and disordered systems, semi and superconducting materials, glasses and amorphous materials, geology, geochemistry, biology, corrosion, high pressure studies, mineralogy etc. Due to this reason XAS has attracted considerable interest among the X-ray spectroscopes. For the structural study of some molecules and compounds in the condensed state Sayers, Lytle, and Stern(1,2,3) made the first quantitative use of XAS about four decades ago although study on the subject has been started since 1920. Further improvements were made in the qualitative understanding of the subject, and the quantitative application of the XAS.(1)
Traditionally, XAS has been divided into two regions: XANES (X-ray absorption near edge structure) Which lies up to approximately ~50 eV above the threshold and EXAFS (Extended X ray absorption fine structure), from ~50 eV above the edge to even 1000 eV above the edge. This division is based on the fact that a number of factors such as multiple scattering (M.S.), chemical effects and the curvature of the outgoing photo electron etc, which are relatively insignificant in the EXAFS region become important in the XANES region. The complexity of these phenomenon makes the analysis of XANES more difficult.
The understanding of EXAFS (Extended X-ray absorption fine structure) has got nearly perfection but many questions are still left unsolved in the field of XANES.(2) This may be due to the general belief that the XANES is very much complex in its origin. In the first half of the 20th Century several attempts were made for the understanding of X-ray absorption spectra through the studies of Petersen, Hayasi, Lindh, Fricke, Kronig, Kostarev and other and Kossel, but probably Natoli was the first in the eighties who initiated for the theoretical understanding of XANES in a right direction. In spite of some modifications and applications in recent years some features of XANES have not been understood well, the present work has been concentrated in this aspect of XANES.(3)
Absorption Process of X-Ray
When the continuous X-rays having energy less than 1,02 MeV is allowed to pass through the absorbing material; absorption of X-ray takes place within the material. The intensity of the transmitted rays from the absorbing material is reduced due to (i) Photoelectric effect (ii) Auger effect and (iii) X-ray scattering effect, etc. These variations of intensity of the transmitted rays are gradual.
But when the energy of the incident X-rays becomes just sufficient to promote one of the inner electrons to an orbit outside the system of the filled orbits (i.e., outside the absorbing atom) the intensity variations become abrupt.(4) The absorption of X-rays is given by the classical exponential law:
![]()
As the wave length of the incident ray increases (i.e. the energy of the incident X-ray decreases) the absorption coefficient increases gradually. But at particular wave lengths an abrupt change in absorption takes place where high absorption occurs. This results into an abrupt change in transmitted intensities.
These abrupt discontinuities are called absorption discontin-unities or absorption edges. Thus the external structure of the atom affects the absorption spectrum. Therefore, the absorption spectrum gives the ideas of the way in which an atom is influenced by the physical and chemical environment of the absorbing atom.
The edge corresponding to the most tightly bound initial state of the photo absorbing atom is known as the K-edge, followed by the L-edge having three components (LI, LII and LIII), in turn followed by the M-edge having five components (MI, MII, MIII, mIV and MV), and so on.(5) These edges correspond to the excitation potentials of the energy levels of the concerned atom.
The absorption edge most commonly is defined in four different ways:
The absorption threshold
It is the minimum level of energy state reached by the core excitation needed for the beginning of X-ray absorption process under the exposure of X-rays.
The absorption jump edge or the rising edge
It is the energy where the absorption co-efficient is at half height of the atomic absorption jump, i.e., the difference between the absorption co-efficient above and below threshold.
The continuum threshold or the ionization threshold
It is the energy where the electron is ejected in the continuum. (Continuum is the vacuum level in metals and the bottom of the conduction band in insulators).
Peaks in derivative spectra
Electrons start travelling from inner energy level to the outer energy level after getting energy from the incident rays. This is equivalent to the transition of holes moving from outer to inner energy level.
X-Ray Absorption Spectra
In X-ray absorption spectra we find strong and sharp peaks just after rising edge as well as weak oscillations at higher energies. Any structure in the spectra observed near the inner shell absorption threshold was called as Kossel structure during the initial period of the development of X-ray absorption spectroscopy.(6) This has been assigned to electron transition to the bound excited state. The structures beyond 20 eV (about) were referred to as Kronig structures.(7) These structures were assigned to the local structure. Low energy range (Kossel) structure was used to determine the electronic structure and the high energy range (Kronig) structure was used to determine the local spatial structure. An experimentally observed spectrum of Molybdenum K- edge has been shown in as an example. The success of one electron single scattering short range order theory brought revival of interest in the Kronig structure and was renamed as Extended X-ray Absorption Fine structure (EXAFS). Presently it is being used as a tool for the local structure determination in the complex material. The energy range of XAS for EXAFS analysis extends above the value of photoelectron wave vector K = 3-4Å-1
We find strong absorption features in XAS when there is a range of photoionisation energy up to ~50eV. These features are known by XANES and are determined by the atomic geometrical arrangements in a local cluster around the absorbing atom via the multiple scattering (M.S.) of the excited photo-electron.
Edge and Main Peaks: Shapes and Their Position
The position and shape of the absorption edge depends upon the nature of ligands and the bonds formed with the absorbing atom. Thus the absorption edge carries information about the molecule itself. The energy position of the edge indicates the effective charge density on the absorber and the shape of edge gives information about the type and symmetry of the ligands. There is a change in the local potential around the absorbing atom according to the type and symmetry of ligands. Therefore, a change in the local potential around the absorbing atom due to the change in the type and symmetry of ligands is observed in the form of shift in the position of the absorption edge called as the chemical shift. The chemical shift ∆E is defined as,
∆E = EC - EM (1)
Where:
EC - refer to the edge position in the compound.
Em - refer to the edge position in the element.
The chemical shift has been related to the effective charge on the absorber. Thus the shift increases with the increase in the formal oxidation state. Many researchers have established correlation between chemical - shift and effective charge.
The transition probabilities and the width of inner levels involved are the two determining factors for the shape of the edge which involve the relative intensities of the peaks. The shape of the edge is directly related to the two aspects of the co - ordination:
Geometry of the Ligand
It influences the dipole allowedness of the transit to bound states and the nature of the continuum shape resonance.
Characteristics of the legends in the bonding at the metallic site
Study of the edge shapes quantitavely has become much difficult here due to inherent complexity in these two aspects of coordination. Therefore, only the qualitative features have been focused till date.
General Feature of Xanes
The local geometry (i.e. the distribution of atoms) including bond angles and relative atomic positions are the characterizing points of XANES. But probing these points by EXAFS in the environment of absorbing atoms is not possible.
On the other hand the continuum XANES features are closer to EXAFS than optical spectroscopy. These features are mainly due to the atomic distribution of neighboring atoms with a minor role of the electronic structure of the system (the distribution of valence electrons in the occupied levels). Moreover, this point where the atomic effects and the electronic structure play the major role introduces a distinction between the edge region (~8eV), and the multiple scattering region.
Importance of Xanes
XANES is strongly sensitive to the chemistry (formal oxidation state and geometry) of the absorbing atom.
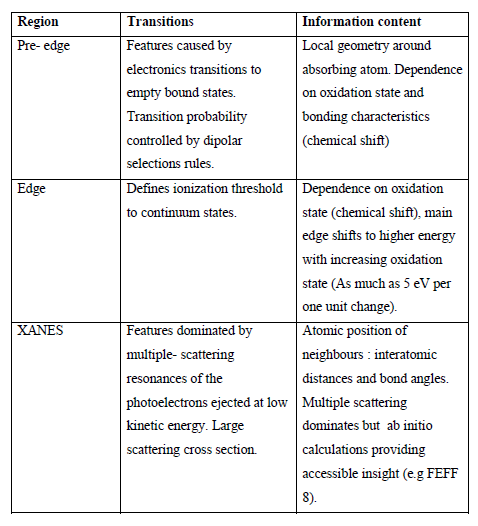
Figure 1. Formal oxidation state and geometry of the absorbing atom
Present Work: Its Orientation
It is belief that XANES is richer in providing structural information around the X-ray absorbing atom than EXAFS attracted the interest of several X-ray spectroscopists.
This is also due to the fact that the decay of the fine structure is rapid past the edge and the several effects such as multiple scattering, many body effects, solid state effects, chemical effects, curved wave effects etc. remain associated with the ejected photoelectron of low kinetic energy and leave their imprint on XAS. The study and analysis of XANES become difficult due to inherent complexity of these phenomena. So many workers on the way of solving these complexities have proposed different models to interpret the origin of various features of XANES. But still some questions are to be answered properly out of which some of them have been mentioned below;
Many workers have proposed several schemes for the determination of energy reference: for XAS, Such as:
(a) First inflection point.
(b) Middle of the sharp rising part of the edge.
(c) First or second peak of the derivative spectra.
(d) Interstitial potential, V0 (proposed by Natoli).
Which is not accessible experimentally.
(e) Energy zero, EO of EXAFS as a free parameter.
In all the above methods, effect of thickness of absorber has not been considered and hence an attempt has been made for the determination of a more suitable energy reference (Characteristic energy S).
We have tried to identify a representative edge, so that some physico - chemical properties of the systems under investigation can be directly measured from the absorption spectra.
The Method suggested by us has been applied to some systems and the correlated with its physico - chemical properties.
We have attempted to investigate the effect of different chemical and physical environment on the main XANES features appearing on the edge.
We have also tried to calculate the nearest neighbor distance of the systems under study by suggesting a simple method.
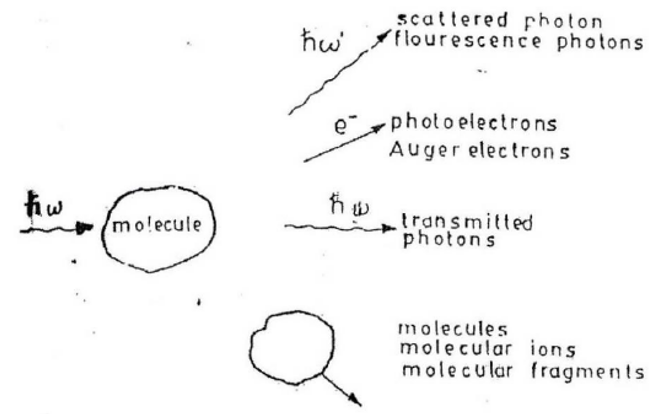
Figure 2. Schematic Representation of the Interaction of Photons with Molecules
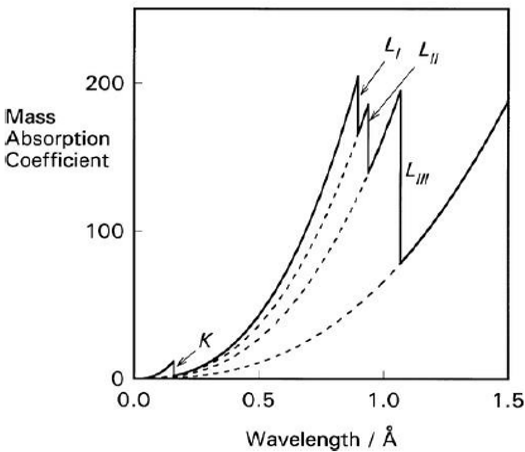
Figure 3. Schematic Illustration of the Variation of Absorption Coefficient with Wave Length
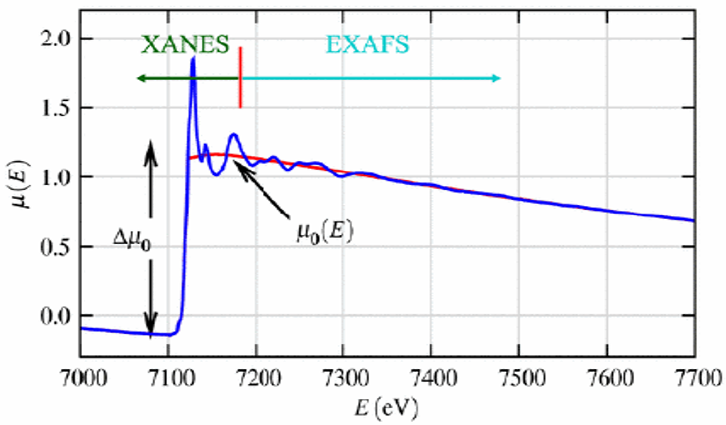
Figure 4. A typical XAFS spectra of FeO (blue) showing the XANES and EXAFS regions as well as the edge-step Δμ0 (E0) and the smooth background function μ0 (E) (red) representing the absorption of an isolated atom
CONCLUSIONS
X-Ray Absorption Spectroscopy is an indispensable tool in modern science, offering unparalleled insights into the local structure and electronic states of materials. Continuous technological innovations and computational advancements are broadening its applicability and enhancing its precision. As research demands evolve, XAS will remain at the forefront of analytical techniques, driving discoveries in diverse scientific fields.
BIBLIOGRAPHIC REFERENCES
1. De Groot F. High-resolution X-ray emission and X-ray absorption spectroscopy. Chem Rev. 2001;101(6):1779–808.
2. Malzer W, Schlesiger C, Kanngießer B. A century of laboratory X-ray absorption spectroscopy–A review and an optimistic outlook. Spectrochim Acta Part B At Spectrosc. 2021;177:106101.
3. Bressler C, Chergui M. Ultrafast X-ray absorption spectroscopy. Chem Rev. 2004;104(4):1781–812.
4. Van Bokhoven JA, Lamberti C. X-Ray Absorption and X-Ray Emission Spectroscopy, 2 Volume Set: Theory and Applications. Vol. 1. John Wiley & Sons; 2016.
5. Sharma A, Singh JP, Won SO, Chae KH, Sharma SK, Kumar S. Introduction to X-ray absorption spectroscopy and its applications in material science. Handb Mater Charact. 2018;497–548.
6. Schnohr CS, Ridgway MC. X-ray absorption spectroscopy of semiconductors. Springer; 2015.
7. Schnohr CS, Ridgway MC. Introduction to x-ray absorption spectroscopy. In: X-ray absorption spectroscopy of semiconductors. Springer; 2014. p. 1–26.
FINANCING
None.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Rakesh Kumar Ranjan.
Data curation: Rakesh Kumar Ranjan.
Formal analysis: Rakesh Kumar Ranjan.
Drafting - original draft: Rakesh Kumar Ranjan.
Writing - proofreading and editing: Rakesh Kumar Ranjan.